Highlights
-
•
A novel report of metastatic ovarian cancer post FIGO stage 1a endometrial adenocarcinoma, without myometrial invasion.
-
•
Metastatic ovarian cancer has been reported with grade 2–3 lesions, myometrial invasion, and extra-uterine involvement.
-
•
Postmenopausal ovarian preservation should be approached with caution for well-differentiated, FIGO stage 1a tumours.
Keywords: Endometrial Cancer, Grade 1 Endometrioid Adenocarcinoma, FIGO Stage 1a, Metastatic Ovarian Cancer
Abstract
Endometrial endometrioid type cancer is a common gynaecological cancer for which the standard surgical management includes hysterectomy and bilateral salpingo-oophorectomy. The value of oophorectomy is to remove occult ovarian disease. It is estimated that 5 % of low grade endometrioid adenocarcinoma will have concurrent ovarian involvement (3 % synchronous tumours, 2 % ovarian metastases), of which only 1 % will be microscopic. Ovarian preservation at the time of surgery can be considered, especially in early-stage disease or premenopausal women. We describe a case of metastatic ovarian disease following surgical management of grade 1 endometrial endometrioid adenocarcinoma confined to the endometrium (FIGO stage 1a), in a postmenopausal woman who declined primary oophorectomy. This case was without genetic predisposition and recurred 12 months after initial surgical treatment. This case is incongruent with what has previously been understood for FIGO stage 1a endometrial endometrioid adenocarcinoma and highlights that even disease seemingly confined to the endometrium can metastasise microscopically to the ovaries.
1. Introduction
Endometrial endometrioid type cancer is the most common gynaecological cancer in developed countries (Morice et al., 2016). Standard surgical treatment includes a hysterectomy, bilateral salpingo-oophorectomy and sentinel node assessment. Ovarian preservation at the time of surgery is sometimes considered, especially in patients with early stage, well differentiated endometrioid cancer of the uterus. Previous reports have suggested that grade 1 endometrioid adenocarcinoma confined to the endometrium is a suitable indication of ovarian preservation if this is requested given the negligible risk of recurrent ovarian disease (Gemer et al., 2004, Modaress et al., 2011). We present a case of a patient with grade 1 endometrial endometrioid adenocarcinoma confined to the endometrium with ovarian preservation at the time of surgery based on patient request. This tumour recurred in the ovary within 12 months and we review the literature with regards to ovarian preservation in patients with endometrial cancer.
2. Case report
A 63-year-old woman was referred to a gynaecologic oncology unit with a grade 1 endometrioid adenocarcinoma on uterine curettings, following intermittent episodes of postmenopausal bleeding. Further investigations revealed no metastatic disease on CT imaging and a normal Ca125 level of 10.0 kU/L (normal range 0.00–35.0 kU/L). She had no additional medical or surgical history and was not taking any medications. Her obstetric history included one vaginal delivery. The patient was advised to have a total laparoscopic hysterectomy, bilateral salpingo-oophorectomy (BSO) and sentinel node assessment. She was counselled that oophorectomy was the standard treatment for her pathology and the need for thorough staging. She declined to have her ovaries removed, or any nodal assessment, and instead underwent a total laparoscopic hysterectomy and bilateral salpingectomy, despite multiple consultations highlighting the deviation from standard treatment protocol. Pelvic washings were not obtained at this time.
The histopathology confirmed a grade 1 endometrioid adenocarcinoma confined to endometrium with positive estrogen receptor (ER) staining (90 %) and progesterone receptor (PR) staining (50 %) on immunohistochemistry. The uterine tumour was not visible macroscopically and no lymphovascular space invasion (LVSI) was seen. There was a loss of MLH1 and PMS2 nuclear staining by immunohistochemistry, and normal staining of MSH2 and MSH6. A subsequent PCR test showed no evidence of microsatellite instability, indicating that immunohistochemistry had detected a sporadic loss of proteins (e.g. promoter hypermethylation), rather than a germline gene mutation (e.g. Lynch Syndrome). The conclusion from a multi-disciplinary meeting was that this was presumed FIGO Stage 1a endometrioid adenocarcinoma (as complete staging was not possible in the setting of ovarian preservation and omission of nodal assessment), and thus no further treatment was recommended. A routine pelvic ultrasound performed 6 months later revealed normal atrophic ovaries.
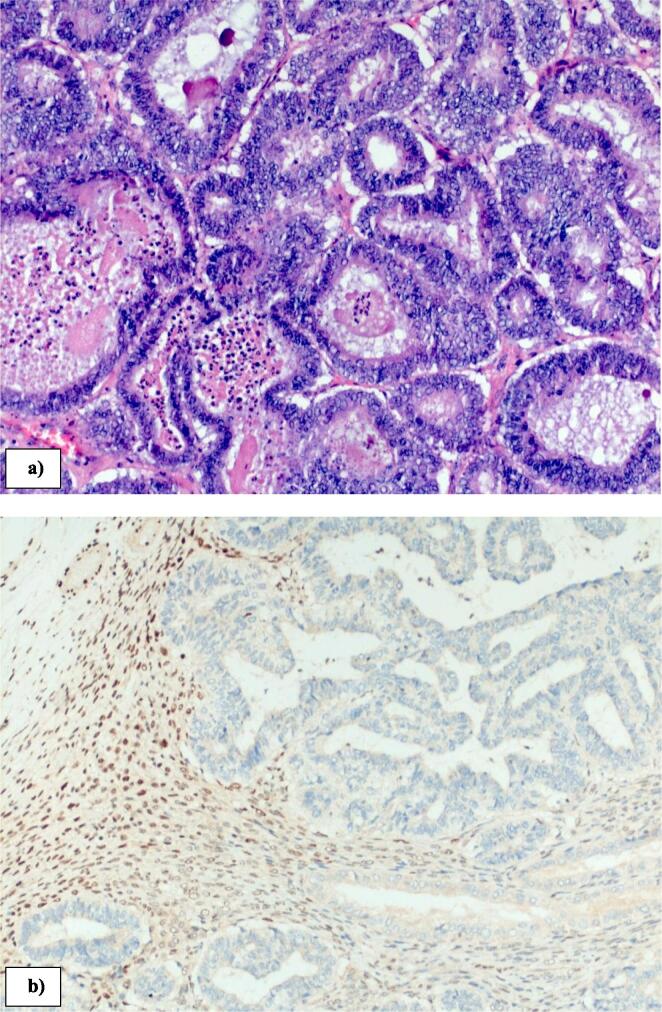
Twelve months after initial surgery she developed right lower abdominal pain and CT scan confirmed a 5 cm multi-cystic, right adnexal mass with no evidence of distant metastatic disease. A serum Ca125 was elevated at 363 kU/L (normal range 0.00–35.0 kU/L). She subsequently underwent a laparotomy, and a large right ovarian mass (macroscopic tumour size 75 × 60 × 40 mm, weighing 76 g) (Fig. 1a) and b)) was noted to be adherent to the omentum and ileocolic bowel. She underwent a bilateral oophorectomy, omentectomy and ileocolic resection with primary anastomosis. There was no evidence of other disease at the time of surgery. Histology confirmed an adenocarcinoma (Fig. 2a) with weakly positive ER (5 %), PR weakly positive (20–30 %). There was no LVSI. Loss of MLH1 (Fig. 2b) and PMS2 was again noted. Pelvic washings were obtained for cytologic evaluation and did not show any evidence of malignancy. The pathologic features were compatible with a recurrent endometrial cancer and the patient underwent 6 cycles of combination carboplatin, paclitaxel followed by adjuvant external beam radiotherapy of 45gy in 25 fractions. The rationale for pelvic radiation therapy was for pelvic control given the high risk of recurrence for a locally advanced endometrial cancer (Creutzberg et al., 2019). She remained asymptomatic and free of disease at 12 months follow-up from her subsequent operation.
Fig. 1.
Right ovarian tumour a) surface appearance; b) macroscopic cross sections.
Fig. 2.
Histopathology of right ovary demonstrating a) grade 1 endometrioid adenocarcinoma (10× magnification, Haematoxylin and Eosin); b) abnormal loss of MLH1 (10× magnification immunohistochemistry).
3. Discussion
We describe a postmenopausal woman with grade 1, and presumed, stage 1a endometrioid adenocarcinoma who, after declining oophorectomy, re-presented with metastatic ovarian disease 12 months later. The value of oophorectomy is the potential to remove occult metastases or synchronous ovarian disease (Gu et al., 2017). In addition to this, there is the theoretical benefit of cessation of ovary-dependent oestrogen production, which could stimulate residual microscopic lesions (Gu et al., 2017). It is estimated that 5 % of low grade endometrioid adenocarcinoma will have concurrent ovarian involvement (3 % synchronous tumours, 2 % ovarian metastases), of which 1 % will be microscopic (Lin et al., 2015). In a retrospective review of 759 women with early-stage endometrial endometrioid adenocarcinoma microscopic ovarian disease was only found in 3 women (0.8 %), all of whom were greater than 50 years of age, with grade 3 disease, deep myometrial invasion and extra-uterine involvement (Lin et al., 2015). Our patient was greater than 50 years of age, however otherwise had none of the features of other cases reported with microscopic ovarian metastases. The authors of this retrospective review concluded that in the setting of ovaries of normal size and appearance ovarian preservation was reasonable for premenopausal women.
In the setting of premenopausal women wishing to preserve their fertility the National Comprehensive Cancer Network (NCCN) describes the ideal candidate as having well-differentiated (grade 1) endometrioid adenocarcinoma on dilatation and curettage confirmed by expert pathology review (Koh et al., 2018). Disease must be limited to the endometrium, preferably confirmed by MRI, or alternatively with transvaginal ultrasound scan. There must be no suspicious or metastatic disease and should be anticipated to be stage 1a (without myo-invasion) (Koh et al., 2018). Meta-analyses of the available data have concluded there is no significant difference in overall survival for premenopausal patients undergoing ovarian preservation compared with BSO (Gu et al., 2017). The recurrent disease noted in our case report suggests that these criteria may not be applicable to postmenopausal patients. In general, there is a paucity of safety data available for ovarian preservation in the post-menopausal group and age remains an important risk factor for metastases, specifically >45 years (Liang et al., 2021). In addition to age, ovarian metastasis risk is also significantly higher for myometrial invasion >1/2, any pelvic lymph node invasion, LVSI, grade 3 lesions, non-endometrioid pathological subtypes and cervical invasion (Liang et al, 2021).
When assessing new ovarian tumours on a background of known endometrioid adenocarcinoma it is important to consider the differential of a synchronous ovarian cancer. Synchronous ovarian disease is thought to be more likely in young, nulliparous, premenopausal and obese women (Oranratanaphan et al., 2008). The pathologic criteria to distinguish ovarian metastasis from a synchronous primary ovarian cancer have been described and includes the presence of precursor lesions, such as ovarian endometriosis (Scully et al., 1998). Despite this helpful histological criteria, recent literature suggests molecular typing of uterine and ovarian disease often show clonality (Schultheis et al., 2016), meaning the true incidence of metastases has probably been underestimated in the literature thus far. Features supportive of metastases in our case include histologic similarity of the tumours at two sites, multinodular surface appearance (Fig. 1a), an absence of endometriosis and similar immunohistochemistry in both tumours (Scully et al., 1998). Although the absence of myometrial and vascular invasion is included in the criteria for a synchronous ovarian primary (Scully et al., 1998), the temporal relationship for disease progression in our case, along with the histologic and genetic features strongly suggested metastatic endometrial cancer. Patients with synchronous ovarian primary disease have a better disease-free survival and overall survival when compared to those diagnosed with metastatic disease (Oranratanaphan et al., 2008).
Deficient mismatch repair (dMMR) function and microsatellite instability is found in 20–30 % of endometrial cancers and hence dMMR testing is now a standard part of pathological workup (Deshpande et al., 2020). Sporadic mutations make up 90 % of dMMR mutations (Deshpande et al., 2020). Delineating between sporadic and germ-line mutations provides important prognostic information and risk profiling for patients, but also contributes to our understanding of clonal relatedness (as in our case) and may hold promise for future therapeutic strategies (Deshpande et al., 2020).
The two pathological pathways proposed for ovarian metastasis are trans-tubal implantation and lymphatic spread (Gemer et al., 2004). Findings consistent with trans-tubal implantation include positive peritoneal cytology, concurrent fallopian metastases, and negative nodal assessment (Gemer et al., 2004). Conversely lymphatic spread rarely has positive peritoneal cytology with concomitantly positive nodes (Gemer et al., 2004). A lymph node assessment was not performed in our case as the patient declined. The incidence of nodal metastasis in patients with grade 1, stage 1a endometrioid carcinomas has been reported as 2.3 % (Pavlakis et al., 2017), however this study did not separate disease confined to the endometrium and disease with < 50 % myometrial invasion. A separate study examined the rate of positive sentinel lymph nodes in the subgroup of grade 1a disease confined the endometrium and found a 0 % rate of micro or macro-metastatic nodal disease, with < 1 % positive for isolated tumour cells (Mueller et al., 2020). The risk of lymph node metastases for grade 1 tumours is significantly increased when the maximal tumour diameter is more than 4 cm and in the presence of LVSI (Pavlakis et al., 2017). It therefore seems very unlikely that lymph node assessment would have changed the initial staging or treatment for our case.
This case is incongruent with what is previously known and understood about stage 1a endometrial endometrioid adenocarcinoma. This case highlights that although ovarian tumours, both synchronous and metastatic, are uncommon in the setting of low grade endometrioid adenocarcinoma, it is possible that even disease seemingly confined to the endometrium can metastasise microscopically to the ovaries. This case highlights the need for caution in preserving the ovaries in postmenopausal patients, even in well differentiated tumours confined to the endometrium.
4. Consent
Written consent was obtained from the patient for publication of this case.
5. Author Contributions Statement
Selvan Pather was responsible for the conceptualisation and supervision of the manuscript. The original manuscript was drafted by Sophia Hill and subsequently reviewed and edited by all authors. All authors have contributed to the synthesis of the discussion and have approved the final version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Creutzberg, C.L., Lu, K.H., Fleming, G.F., 2019. Uterine cancer: adjuvant therapy and management of metastatic disease. J. Clin. Oncol. https://doi.org/10.1200/JCO.19.00037. [DOI] [PubMed]
- Deshpande M., Romanski P.A., Rosenwaks Z., Gerhardt J. Gynecological cancers caused by deficient mismatch repair and microsatellite instability. Cancers. 2020;12:3319. doi: 10.3390/cancers12113319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemer, O., Bergman, M., Segal, S., 2004. Ovarian metastasis in women if clinical stage I endometrial carcinoma. Acta Obstet. Gynecol. Scand. 83, 208–210. https://doi.org/10.1111/j.0001-6349.2004.00408.x. [DOI] [PubMed]
- Gu H., Li J., Gu Y., Tu H., Zhou Y., Liu J. Survival impact of ovarian preservation on women with early-stage endometrial cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer. 2017;27:77–84. doi: 10.1097/IGC.0000000000000857. [DOI] [PubMed] [Google Scholar]
- Koh W., Abu-Rustum N.R., Bean S., Bradley K., Campos S.M., Cho K.R., et al. Uterine Neoplasms, Version 1, 2018. J. Natl. Compr. Canc. Netw. 2018;16(2):170–199. doi: 10.6004/jnccn.2018.0006. [DOI] [PubMed] [Google Scholar]
- Liang X., Zneg H., Chen S., Jiang M., Si L., Fan J. Ovarian metastasis risk factors in endometrial carcinoma: A systematic review and meta-analysis. Euro J. Obs & Gyne Reproductive Bio. 2021;267:245–255. doi: 10.1016/j.ejogrb.2021.11.016. [DOI] [PubMed] [Google Scholar]
- Lin K.Y., Miller D.S., Bailey A.A., Andrews A.J., Kehoe S.M., Richardson D.L., et al. Ovarian involvement in endometrioid adenocarcinoma of the uterus. Gynec Onc. 2015 doi: 10.1016/j.ygyno.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Modaress M.G., Cheraghi F., Zamani N. Ovarian metastasis in endometrioid Type endometrial cancer. Int. J. Fertil. Steril. 2011;5(3):148–151. [PMC free article] [PubMed] [Google Scholar]
- Morice P., Leary A., Creutzberg C., Aub-Rustum N., Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- Mueller J.J., Nobre S.P., Braxton K., Alektiar K.M., Leitao M.M., Aghajanian C., et al. Incidence of pelvic lymph node metastasis using FIGO staging and sentinel lymph node mapping with ultrastaging in surgically staged patients with endometrioid and serous endometrial carcinoma. Gynae Oncol. 2020;157:619–623. doi: 10.1016/j.ygyno.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranratanaphan S., Manchana T., Sirisabya N. Clinicopathologic variables and survival comparison of patients with synchronous endometrial and ovarian cancers versus primary endometrial cancer with ovarian metastasis. Asian Pacific J. Cancer Prev. 2008;9:403–408. [PubMed] [Google Scholar]
- Pavlakis K., Rodolakis A., Vagios S., Voulgaris Z., Messini I., Yiannou P., Vlachos A., Panoskaltsis T. Identifiable risk factors for lymph node metastases in grade 1 endometrial carcinoma. Int. J. Gynae Cancer. 2017;27(8):1694–1700. doi: 10.1097/IGC.0000000000001070. [DOI] [PubMed] [Google Scholar]
- Schultheis, A.M., Ng, C.K.Y., De Filippo, M.R., Piscuoglio, S., Macedo, G.S., Gatius, S., et al., 2016. Massively parallel sequencing-based clonality analysis of synchronous endometrioid endometrial and ovarian carcinomas. JNCI Natl. Cancer Inst. 108(6), djv427, https://doi.org/10.1093/jnci/djv427. [DOI] [PMC free article] [PubMed]
- Scully R.E., Young R.H., Clement P.B. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament”, Washington Armed Forces Institute of Pathology. Atlas Tumor Pathol. 1998;18(3):288. [Google Scholar]