Figure 2. Simplified models of the sex determination pathway in male (A) and female (B) Aedes mosquitoes.
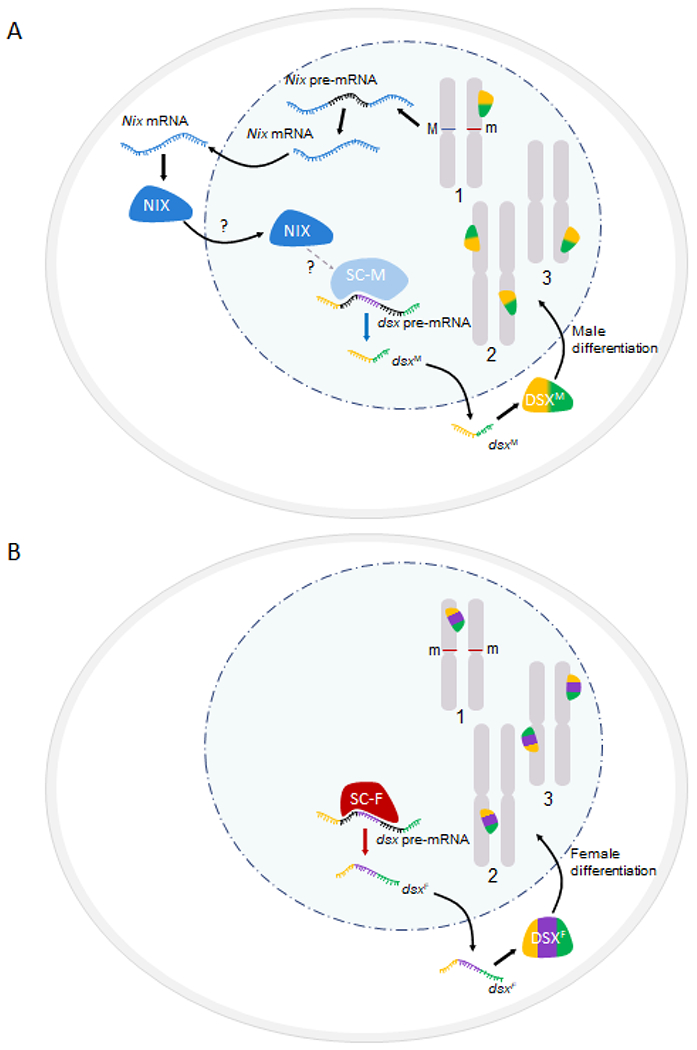
Events leading to the sex-specific splicing of the dsx pre-mRNA are depicted based on information from Ae. aegypti and Ae. albopictus. Details on possible activation or repression of certain splice sites can be found elsewhere [12, 33, 98]. A). Nix, which is located in the M locus of the M/m males, is transcribed, processed, and translated in the early embryo. The NIX protein has predicted RNA recognition motifs and is distantly related to a splicing factor TRA2 [24]. Although we cannot rule out a role in regulating translation or mRNA stability, NIX is hypothesized to function as a splicing factor that somehow modulates an uncharacterized splicing complex in males (SC-M), ultimately affecting dsx splicing. It appears that the effect of NIX on dsx splicing is indirect [33]. The dashed arrow and question mark indicate the uncertainty about the number of steps and the mechanism involved in this modulation. The resulting male DSXM protein isoform participates in programming male differentiation. B) Females (m/m) do not have the M locus and do not express Nix. An uncharacterized splicing complex in the female (SC-F) is responsible for the female-specific splicing of dsx pre-mRNA to produce the dsxF mRNA, which is translated in the cytoplasm to make the female DSXF protein isoform. The DSXF protein is a transcription factor that programs female differentiation through the regulation of gene expression in the nucleus.
