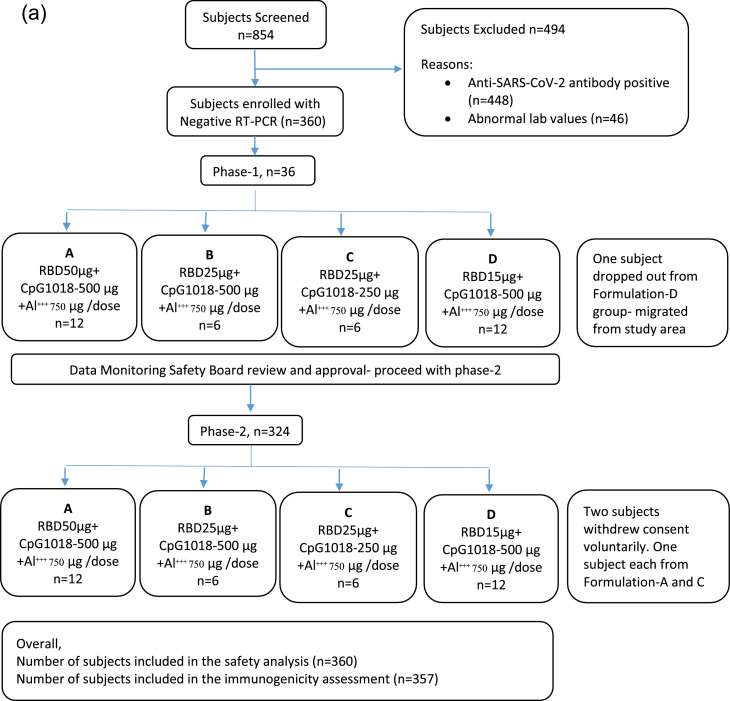
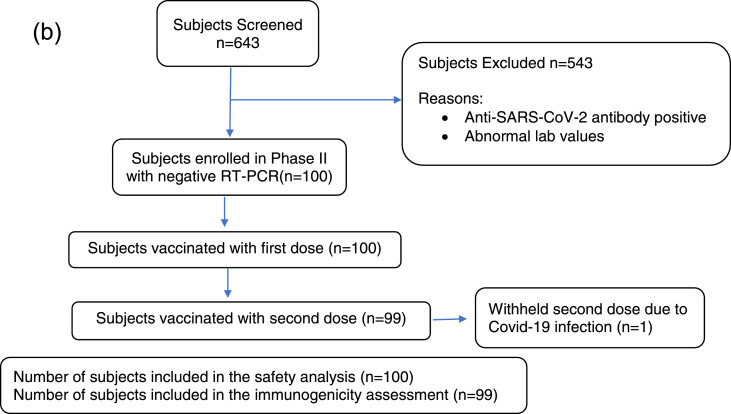
Figure 1.
(a) Subject disposition in Phase-1/2 study (consort diagram). A total of 854 subjects were screened, and 360 subjects were randomly randomised to four different formulation groups (n=90 in each group). All subjects were of Indian origin; 305 (84.72%) were male the mean ±SD age was 34.4±8.26 years and BMI (kg/m2) was 24.8±3.03. All subjects received the first dose, and 358 subjects received the second dose of the COVID-19 vaccine of Biological E. Three participants voluntarily withdrew from the study. A total of 357 (99.17%) and 360 (100%) patients were included in the immunogenicity and safety analyses, respectively.
N, number; RT-PCR, reverse transcription polymerase chain reaction; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
(b) Subject disposition in Phase-2 part of Phase-2/3 study. A total of 643 subjects were screened, and 100 were enrolled in the study. All 100 subjects were of Indian origin and received the first dose, and 99 subjects received the second dose of COVID-19 vaccine of Biological E. Out of the 100 subjects, 86 (86.00%) were male, and the mean ±SD age was 33.2 ±8.41 years. In one subject, the second dose of the vaccine was withheld owing to COVID-19 infection. A total of 99 (99%) and 100 (100%) patients were included in the immunogenicity and safety analyses, respectively.
n, number; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.