Abstract
The past decade has witnessed a change in landscape of cancer management with the advent of precision oncology. Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment and have played an important role in improving patient survival. While the patients are living longer, treatment with ICIs are sometimes associated with adverse effects, some of which could be fatal. Radiologists can play a crucial role by early identification of some of these adverse effects during restaging scans. Our paper focuses on the imaging features of commonly occurring ICI toxicities based on organ system.
Keywords: Immune check point inhibitors toxicity, Pneumonitis, Colitis, Pancreatitis, Hepatitis
Abbreviations: ICI, Immune check point inhibitor; CTLA-4 inhibitor, Cytotoxic T-lymphocyte antigen- 4 inhibitor; PD-L1 inhibitor, programmed cell death ligand-1 inhibitor; PD-1 inhibitor, programmed cell death-1 inhibitor; irAE, immune-related adverse event; CTCAE, Common Terminology Criteria for Adverse Events; PFS, progression free survival; RRP, radiation recall pneumonitis; FDA, Food and Drug Administration; NSCLC, non-small cell lung cancer; OP, organizing pneumonia; NSIP, non-specific interstitial pneumonia; AIP, acute interstitial pneumonitis; ARDS, acute respiratory distress syndrome; LGE, late Gadolinium enhancement
1. Introduction
The discovery of immune checkpoint inhibitors (ICIs) brought a paradigm shift in cancer management. The first ICI approved by the US Food and Drug Administration (FDA) in 2011 was Ipilimumab to treat metastatic melanoma [1]. Based on their mechanism of action, three major types of ICIs have been identified: Cytotoxic T-lymphocyte antigen- 4 inhibitor (CTLA-4 inhibitor), programmed cell death-1 inhibitor (PD-1 inhibitor), and programmed cell death ligand-1 and 2 inhibitors (PD-L1 and PD-L2 inhibitors) [2], [3]. Currently, FDA-approved ICIs include Ipilimumab (CTLA-4 inhibitor); Nivolumab, Pembrolizumab, and Cemiplimab (PD-1 inhibitors); and Atezolizumab, Durvalumab, and Avelumab (PD-L1 inhibitors) [4]. ICIs have been mainly approved for the treatment of advanced solid and hematological malignancies [4]. Additionally, FDA has also approved ICIs as the first-line therapy for some cancers like microsatellite instability-high or mismatch repair deficient colorectal cancer. ICIs are used either as monotherapy or as combination therapy termed “dual ICIs” or in combination with chemotherapy, so-called “chemoimmunotherapy.”
While these newer treatment agents have improved patient survival, they can be associated with adverse effects, ranging from abnormal laboratory tests or asymptomatic imaging findings to severe cases that can result in death. The negative effects or complications are termed “immune-related adverse events (irAE).” The incidence rate of irAEs can range from 30− 66% [5]. Studies have shown that irAE development is associated with improved efficacy of ICI, improved patient survival, and enhanced response rates [6], [7]. A prior study by Toi et al. in patients with non-small cell lung cancer (NSCLC) treated with Nivolumab showed that patients who experienced irAEs had a more prolonged median progression-free survival (PFS) of 12 months while patients without irAEs had a shorter median PFS of 3.2 months [6]. Therefore, early identification of irAE is key to continuing ICI therapy while managing the adverse effects in their early stages. This can be accomplished by modifying the therapy or starting supportive or mitigation measures such as steroid administration.
To standardize the reporting of adverse effect from anticancer agents, including the irAEs, the National Cancer Institute (NCI) developed specific terminology termed the “Common Terminology Criteria for Adverse Events (CTCAE), which helps in grading the severity of the events into 5 categories, [8].” Grade 1 includes asymptomatic toxicity which can be visible on imaging or toxicity with mild symptoms which do not require treatment, while grade 5 includes very severe cases which result in death [8]. Please refer to Table 1 for CTCAE classification [8]. CTLA-4 inhibitors are associated with more severe irAEs compared to PD-1/PD-L1 inhibitors. In a prior study, CTLA-4 inhibitors showed a greater incidence of grade 3 or higher irAEs (33%) compared to PD-1 inhibitors (10%) [5]. The incidence of irAEs is higher with combined therapy when compared to monotherapy with ICIs.
Table 1.
CTCAE (Common terminology criteria for adverse events) for grading of irAE (immune-related adverse events).
| Grade | Clinical features | Treatment |
|---|---|---|
| Grade 1 | Asymptomatic or mild symptoms | Observation |
| Grade 2 | Moderate in severity, limiting instrumental activities of daily life | Low dose steroids, ICI maybe suspended temporarily when considered necessary |
| Grade 3 | Severe symptoms limiting activities of daily life but not life threatening | High dose steroids with temporary or permanent suspension of ICI, hospitalization maybe indicated |
| Grade 4 | Severe life-threatening consequences | High dose steroids with discontinuation of ICI, hospitalization is indicated |
| Grade 5 | Death | N/A |
ICI-immune check point inhibitor
Radiologists must be aware of commonly occurring irAEs, specific irAEs associated with certain ICIs, and general timeframes to the onset of irAEs after starting therapy in order to improve their detection on imaging. For the convenience of our readers, we have classified irAEs based on organ systems and described their important clinical features and key imaging findings.
2. Thoracic irAEs
2.1. Pneumonitis
Drug-related pneumonitis is one of the major adverse events in patients receiving immunotherapy [9]. Pneumonitis is focal or diffuse inflammation of the lung parenchyma and is a result of immune-mediated injury, oxidative stress, or cytotoxic response [10]. Pneumonitis is the most common adverse effect of immunotherapy with an incidence rate of 4.1–19% with PD-1/PD-L1 checkpoint inhibitors [11], [12]. The rate is higher when combination therapy with PD-1/PD-L1 inhibitors and CTLA-4 inhibitors is used [11]. The lungs respond to these insults through different mechanisms, which on imaging have the appearance of interstitial pneumonias. Pneumonitis should be suspected when a patient started on immunotherapy complains of shortness of breath, cough, or chest pain. After starting immunotherapy, the median time to onset of pneumonitis is 2.6 months (0.5–11.5 months) [13]. The median time to onset is shorter when combination therapy is used [11]. Some patients with pneumonitis are now successfully treated with steroids and are able to continue with immunotherapy [14], [15]. Severe cases can be life-threatening, require hospital admission with intubation, and may result in death [14], [15]. The mortality rate can be as high as 20% [15]. Imaging plays a key role in detecting and monitoring patients with pneumonitis.
On plain radiographs, pneumonitis often presents as bilateral airspace opacities. On CT, organizing pneumonia (OP) is the most commonly seen pattern of interstitial pneumonia associated with pneumonitis [13]. There are multifocal subpleural and peribronchovascular ground glass or consolidative opacities in the bilateral lungs [13]. A reversed halo appearance in which there is a central ground-glass opacity surrounded by peripheral consolidation can be seen with OP, called “Atoll’s sign” (Fig. 1) [13]. Non-specific interstitial pneumonia (NSIP) pattern is seen in some cases, demonstrating peripheral reticulations with ground-glass opacities or consolidations with sub-pleural sparing and traction bronchiectasis [13]. Hypersensitivity pneumonitis is characterized by upper lobe predominant ground-glass opacities with centrilobular nodules and lobular areas of air trapping [13]. Acute interstitial pneumonitis (AIP) pattern is rarely encountered, and the imaging features are similar to acute respiratory distress syndrome (ARDS) with bilateral ground glass and consolidative opacities in an apicobasal gradient [13]. Lower lobes show dense consolidations while the upper lobes have ground-glass opacities with traction bronchiectasis (Fig. 2) [13]. On PET/CT, pneumonitis presents as FDG-avid bilateral consolidations. In some rare cases, pneumonitis flare is seen [9]. Pneumonitis flare is the recurrence of pneumonitis after the initial episode of pneumonitis is successfully treated and steroid administration is discontinued [9]. This can happen with or without rechallenge with the offending drug. On imaging, pneumonitis flare presents with similar but more extensive opacities in the bilateral lungs and is more severe than the initial presentation [9].
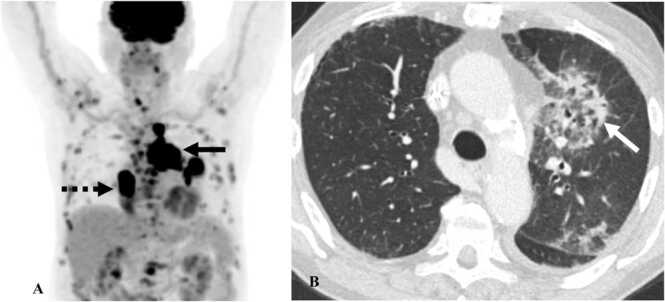
Fig. 1.
Immune-mediated pneumonitis in a 78-year-old man with small-cell lung cancer on Atezolizumab therapy. A. MIP image from PET/CT shows extensive hilar and mediastinal lymphadenopathy (black arrow) and right lower lobe lung mass (dashed black arrow). B. Contrast-enhanced axial chest CT image shows the development of new consolidation with central ground glass opacities (Atoll’s sign) in peribronchovascular distribution (white arrow) consistent with organizing pneumonia pattern of pneumonitis.
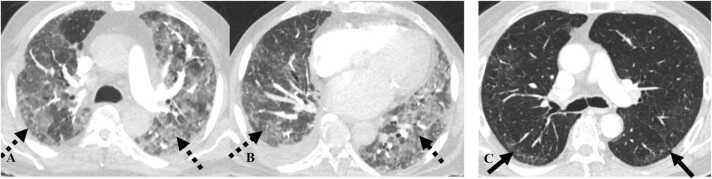
Fig. 2.
Immune-mediated pneumonitis. A&B. Contrast-enhanced axial chest CT images in a 63-year-old man with non-small-cell lung cancer on pembrolizumab show bilateral ground glass opacities with mild traction bronchiectasis (dashed black arrows) representing acute interstitial pneumonia or acute respiratory distress pattern of pneumonitis. C. Contrast-enhanced axial chest CT image in a 78-year-old man with melanoma on pembrolizumab shows peripheral reticulations with subpleural sparing (black arrows) consistent with NSIP pattern of pneumonitis.
2.2. Sarcoidosis-like reaction
Sarcoidosis-like reaction is most commonly seen with ipilimumab (5–7%) though cases have been reported with nivolumab and pembrolizumab (<0.5%) [16], as well as other targeted therapies. The median time to onset of sarcoidosis-like-reaction from initiation of immunotherapy is approximately 14 weeks, ranging from 3 weeks to 2 years [16]. Sarcoidosis-like-reaction can be challenging to diagnose as the imaging features overlap with features of the natural progression of cancer. However, sarcoidosis-like-reaction has a characteristic distribution and either improves or resolves over a period when immunotherapy is continued [16]. Though not routinely indicated, tissue biopsy remains the gold standard for distinguishing sarcoidosis-like-reaction from tumor progression [17]. Steroid administration is reserved for symptomatic cases when patients present with fatigue, fever, or shortness of breath. Mediastinal nodes are the most common site of involvement, followed by skin, lungs, extrathoracic nodes, spleen, CNS, eye, and bone.
On CT, the most classic pattern consists of mediastinal and hilar lymphadenopathy with or without bilateral pulmonary nodules (Fig. 3) [18]. Ground glass opacities are uncommon but can be seen [18]. The abdomen is the most common extrathoracic site of involvement. Splenic involvement presents as splenomegaly with small hypodense lesions in the spleen [19]. In rare cases co-existing intra-abdominal lymphadenopathy may be seen. Interstitial nephritis presents with striated nephrogram on CT with alternating hypodense and hyperdense areas [20]. Pituitary involvement is best diagnosed on MRI with diffuse enlargement of the pituitary gland and infundibulum with intense enhancement on post contrast images and restriction on diffuse weighted imaging [21]. On PET/CT, there is FDG uptake in the involved lymph nodes and tissues (Fig. 3).
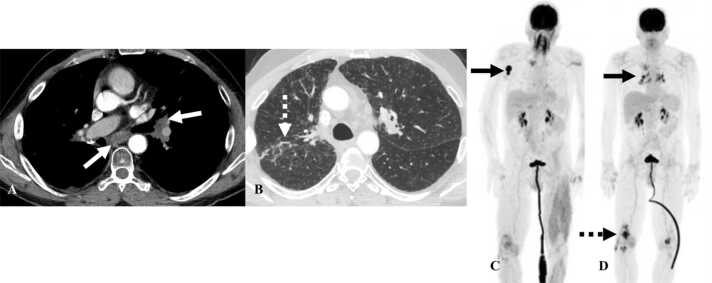
Fig. 3.
Sarcoidosis-like reaction. A&B. Contrast-enhanced axial chest CT images in a 57-year-old man with lung cancer 9 months after initiation of pembrolizumab show mediastinal and hilar lymphadenopathy (white arrows) and innumerable perilymphatic nodules on lung windows (dashed white arrow). C. Reconstructed MIP image from PET/CT in a 54-year-old man with melanoma shows FDG-avid right axillary lymphadenopathy (black arrow), consistent with biopsy proven melanoma. D. Reconstructed MIP image from PET/CT obtained 6 months after the initiation of Nivolumab and Ipilimumab shows resolution of sites of melanoma but new FDG-avid mediastinal and hilar lymphadenopathy (black arrow) consistent with sarcoidosis-like-reaction. Also note inflammatory arthritis in the bilateral knees (dashed black arrow).
2.3. Radiation recall pneumonitis
Radiation recall pneumonitis (RRP) is a very rare complication known to occur with conventional anticancer agents, such as gemcitabine, targeted therapy and immunotherapy [22]. Radiation induced inflammatory changes in the lung subside around 6–9 months after radiotherapy [22]. In RRP, new inflammatory changes occur in the previously radiated lung field after a prolonged quiescent stage triggered by the administration of systemic therapy [23]. Prior case reports have revealed that the time interval from prior radiotherapy to the onset of RRP in patients who received immunotherapy averaged about 2 years [24]. On CT, new ground glass or consolidative opacities are seen at or around an area of prior radiation which has remained quiescent for several months or years after radiotherapy (Fig. 4) [22]. Organizing pneumonia pattern is the most common pattern of pneumonitis associated with RRP [22]. Corticosteroids are the mainstay of treatment and angiotensin converting enzyme inhibitor is used for symptomatic relief [25], [26].
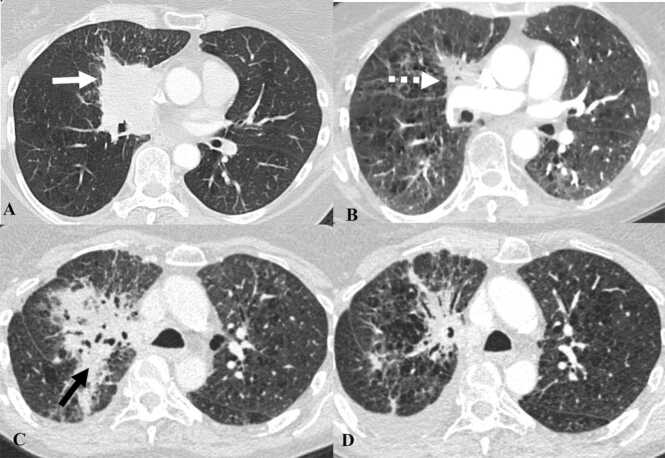
Fig. 4.
Immune-mediated radiation recall pneumonitis in a 65-year-old man with non-small cell lung cancer treated with Durvalumab therapy. Contrast-enhanced axial chest CT images demonstrate the following: A. Right perihilar mass consistent with biopsy proven non-small cell lung cancer (white arrow). B. Patient was treated with radiation and chemotherapy (cistplatin+etoposide) after which the primary tumor decreased in size (dashed white arrow). C. The patient was started on Durvalumab therapy after 2 years with worsening consolidation at the site of prior radiation in the right perihilar region (black arrow), suspicious for radiation recall pneumonitis. D. Durvalumab was stopped, and the patient was placed on steroids after which the pneumonitis decreased.
3. Cardiovascular irAE
3.1. Myocarditis
The incidence of myocarditis is low and ranges from 0.04% to 1.14% however carries a very high mortality rate ranging from 25% to 50% [27], [28], [29], [30]. The average duration to the onset of myocarditis from the start of therapy is 30–60 days [27], [28]. Though the exact mechanism of myocarditis has yet to be determined, the proposed mechanism is a shared antigen between the tumor and the myocardium leading to molecular mimicry resulting in T-cell infiltration of the myocardium [27]. Myocarditis can be asymptomatic with abnormal electrocardiogram and elevated cardiac biomarkers (Troponin I and B-type natriuretic peptide when associated with heart failure) [28], [31]. It usually manifests with chest pain, dyspnea, fatigue, or palpitations when symptomatic. In more severe cases, arrhythmia, heart block, or ventricular tachycardia can occur and result in cardiogenic shock [27], [28]. Endomyocardial biopsy establishes the diagnosis [32]. It is treated by cessation of immunotherapy and administering steroids.
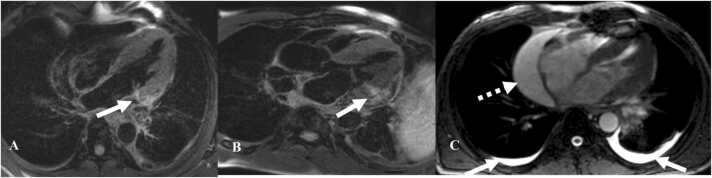
Cardiac MRI is the imaging modality of choice for myocarditis. T2 hyperintensity in the myocardium and late gadolinium enhancement (LGE) is characteristic of myocarditis [33]. Other supportive findings are the presence of pericardial effusion or abnormal signal intensity in the pericardium on T2 images or the presence of LGE (Fig. 5) [33]. Regional or global wall motion abnormalities can be seen with decreased left ventricular ejection fraction in some cases [33]. On cardiac protocol PET/CT, FDG-avidity localized to the myocardium is usually diffuse and patchy in distribution.
Fig. 5.
Immune-mediated myocarditis in a 67-year-old man with non-small cell lung cancer treated with Durvalumab. A&B. Post contrast axial MRI images show enhancement of the lateral and basal part of the left ventricle (white arrows) C. T2WI shows pericardial effusion along the right heart border (dashed white arrow) and small bilateral pleural effusions (white arrows).
3.2. Pericardial disease
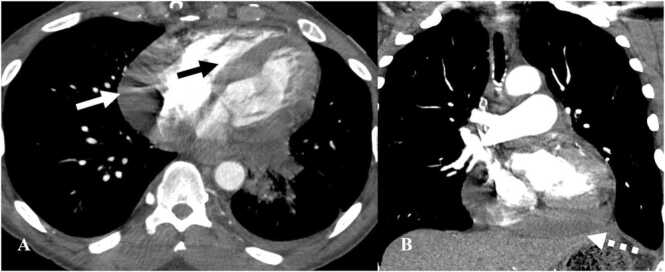
Pericardial involvement is a less frequently seen adverse effect of ICI therapy [34] and may manifest with pericarditis, pericardial effusion, or tamponade [34]. Pericarditis is seen as enhancement of the pericardium on contrast-enhanced CT. Pericardial effusions are typical, with variable amount of fluid in the pericardial space. When a large amount of fluid accumulates in the pericardium over a short amount of time, it can result in tamponade (Fig. 6). Tamponade is increased pressure within the pericardium associated with the collapse of the right ventricle, bowing of the interventricular septum, enlargement of superior venacava, inferior venacava (IVC), hepatic, and renal veins, reflux of contrast into the IVC or azygos vein. Tamponade is an emergency, and treatment is immediate pericardiocentesis.
Fig. 6.
Immune-mediated pericarditis in a 67-year-old man with non-small cell lung cancer treated with Durvalumab. A. Contrast-enhanced axial chest CT image shows pericardial effusion along the right heart border (white arrow). Note the straightening of interventricular septum which can be seen with tamponade (black arrow). B. Coronal image in the same patient shows a large pericardial effusion at the base of the heart (dashed white arrow).
3.3. Vasculitis
Vasculitis is a rare complication of immunotherapy with less than 1% incidence rate [35]. Large-vessel vasculitis is more common with features of giant-cell arteritis (GCA) [36], [37]. CT or MR angiography is the diagnostic modality of choice to diagnose vasculitis. There is diffuse circumferential thickening of the vessel wall with mural enhancement or thrombi (Fig. 7). When vasculitis involves the aorta, it can extend into the origin of vessels arising from the aorta such as the subclavian artery, left common carotid artery, superior mesenteric artery, or renal arteries [38]. On PET/CT, FDG-avidity is seen along the wall of the vessel. Less commonly, vasculitis can occur in end-organ arteries causing damage to the involved organ. Vasculitis involving arteries of the jejunum can present with imaging features of occlusion of branch of superior mesenteric artery (SMA) without a true SMA occlusion. For instance, on CT there can be focal dilatation of the jejunum with the enhancement of the mucosa and wall thickening [39]. Vasculitis is usually treated with steroids.
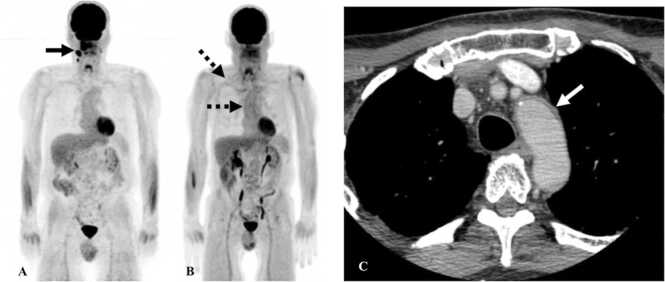
Fig. 7.
Immune-mediated vasculitis in a 67-year-old man with squamous cell cancer of the oropharynx on Nivolumab. A. Reconstructed MIP image from PET/CT shows FDG-avid lesion in the floor of mouth with lymphadenopathy (black arrow). B. Reconstructed MIP image from PET/CT obtained 6 months after initiating Nivolumab therapy shows resolution of the lesion with new FDG uptake along the walls of the aorta and subclavian arteries consistent with vasculitis (black dashed arrow). C. Contrast-enhanced CT of the neck from the same day shows wall thickening of the arch of aorta (white arrow).
4. Gastrointestinal irAE
4.1. Colitis
The incidence of colitis ranges from 8-22% (3% with PD1 inhibitors, 6% with CTLA-4 inhibitors, and 9% with combined therapy) [40], [41]. The median time to onset of colitis from the initiation of immunotherapy is approximately 8 weeks (2–4 months) [42]. Patients present with pain abdomen and diarrhea. Diffuse colitis is treated with steroids alone, but focal colitis is treated with steroids and antibiotics. Infliximab and vedolizumab are used in steroid refractory cases [43], [44].
Two different types of imaging features have been described on CT: diffuse colitis and segmental colitis [42]. Diffuse colitis is characterized by the involvement of a long segment of colon with mucosal enhancement, bowel wall thickening, fluid in the bowel lumen, and mesenteric vessels engorgement (Fig. 8) [42]. Focal colitis is seen in a bowel segment with pre-existing diverticulosis [42]. In focal colitis there is a short segment bowel wall thickening with mucosal enhancement and pericolonic fat stranding [42]. On PET/CT there is FDG uptake within the mucosa of the involved segment of the colon.
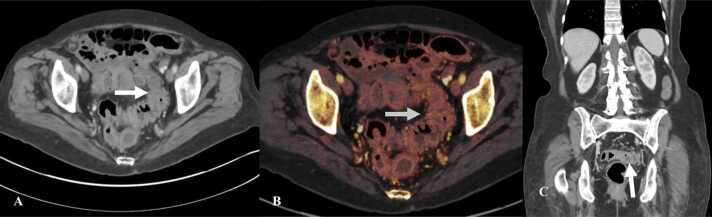
Fig. 8.
Immune-mediated colitis in a 73-year-old woman with renal cell cancer on Nivolumab. A. Contast-enhanced axial CT of the abdomen and pelvis shows wall thickening and enhancement of the descending and sigmoid colon (arrow) with adjacent fat stranding. B. Color overlay image on dual energy CT shows enhancement in the mucosa of sigmoid colon (arrow). C. Coronal image of the abdomen and pelvis shows wall thickening and enhancement of the mucosa of sigmoid colon (arrow).
4.2. Gastroenteritis
Gastritis and enteritis have rarely been reported and occur less frequently than colitis, with few cases described. Gastritis can occur synchronously with Helicobacter or Cytomegalovirus virus infection [45]. The median time to onset of gastritis is around 4–9 months after initiation of ICI therapy [46], [47]. Enteritis has been reported to occur within one month of starting immunotherapy [48]. Patients may complain of pain abdomen, vomiting, nausea, or weight loss. Treatment includes cessation of the offending ICI with steroid administration [46].
On CT imaging, gastritis is seen as diffuse gastric wall thickening with perigastric fat stranding [47]. Endoscopy can confirm the diagnosis and shows mucosal erythema with ulcers. Enteritis is characterized by diffuse wall thickening of the small bowel with mucosal enhancement, distension of the lumen with fluid, and adjacent fat stranding [48]. Diffusely increased FDG-uptake is seen in the walls of the involved bowel segments.
5. Hepatopancreaticobiliary irAE
5.1. Hepatitis
Hepatotoxicity occurs in 1–17% of patients on ICI therapy [49]. The incidence rate is higher with combination therapy. The median time to onset of hepatotoxicity after starting ICI therapy is about 6–14 weeks [50]. The majority of cases present with asymptomatic elevation of liver enzymes (alanine aminotransferase and aspartate aminotransferase). In symptomatic patients, clinical features include pain in the right upper quadrant, jaundice, pruritis, abdominal distension due to ascites, confusion progressing to coma in hepatic encephalopathy. Corticosteroids are the first line therapy [51]. Azathioprine and Tacrolimus are reserved for patients who do not respond to steroids [52].
Imaging features of immunotherapy-related hepatitis are identical to acute hepatitis. Ultrasound shows hepatomegaly and diffusely hypoechoic liver parenchyma with hyperechoic portal triads/periportal edema (“starry sky” appearance) often accompanied by gallbladder wall thickening. CT shows hepatomegaly with hypoattenuation of the hepatic parenchyma with periportal edema, as evidenced by decreased attenuation around the portal system (Fig. 9) [50]. Co-existing periportal lymphadenopathy can also be seen. On MRI, there is increased parenchymal signal intensity on T2-weighted images with increased signal intensity around the portal system consistent with periportal edema with delayed periportal enhancement (Fig. 9).
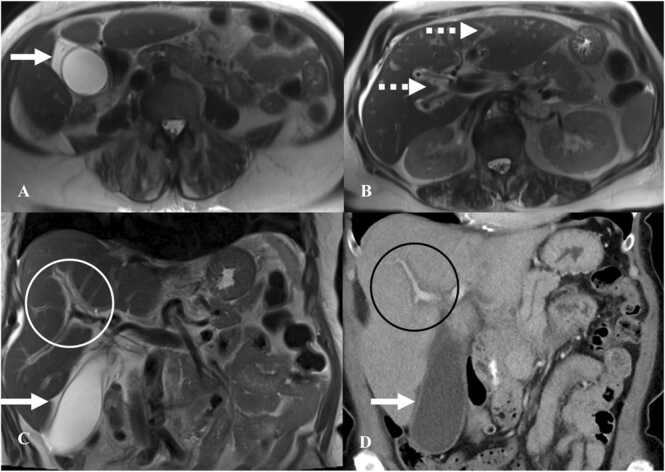
Fig. 9.
Immune-mediated hepatitis in a 68-year-old man with melanoma on Pembrolizumab. A&B. Axial T2W images of MRI of the abdomen show gallbladder wall edema (white arrow) and periportal edema (dashed white arrow). C. Coronal T2W MRI abdomen image shows periportal edema (white circle) and gallbladder wall edema (arrow). D. Coronal image on contrast-enhanced CT of the abdomen shows periportal edema (black circle) with gallbladder wall edema (arrow). Also note diffuse hypoattenuation of the liver.
5.2. Pancreatitis
The incidence rate of ICI-related pancreatitis ranges from 2-8% (2% with CTLA-4 inhibitor, 4% with PD-1/PD-L1 inhibitor, and 8% with combination therapy) [53]. The median time from immunotherapy initiation to the onset of pancreatitis ranges from 2 to 5 months (69 days for CTLA-4 inhibitor, 146 days for PD-1/PD-L1 inhibitor, and 110 days for combination therapy) [53]. The majority of patients are asymptomatic with elevation of serum lipase levels [53]. Symptomatic patients present with epigastric pain, nausea, vomiting, fever, and diarrhea. ICI-related pancreatitis is treated by cessation of the offending ICI therapy, steroid administration, and fluid resuscitation [53].
On CT, diffuse pancreatitis presents as diffuse enlargement of the pancreas with heterogenous enhancement, and peripancreatic stranding (Fig. 10) [54]. In focal pancreatitis there is segmental hypoenhancement with peripancreatic stranding [54]. Complications such as pancreatic necrosis and hemorrhage are rare but reported. Pancreatic atrophy was seen as a chronic sequela in one study [54]. Increased FDG-avidity in and around the pancreas can be seen on PET/CT.
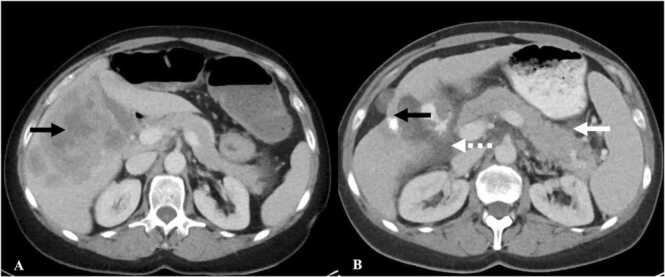
Fig. 10.
Immune-mediated pancreatitis in a 55-year-old man with melanoma on Pembrolizumab. A. Contrast-enhanced axial CT abdomen image shows numerous hepatic metastases (black arrow) with normal appearing pancreas. B. Contrast-enhanced axial CT abdomen image after the initiation of Pembrolizumab shows decrease in the hepatic metastases with liver capsule retraction (black arrow). Note that the pancreas is now bulky with peripancreatic stranding (white arrow) and fluid in the pancreaticoduodenal groove (dashed white arrow) suggesting pancreatitis.
5.3. Cholangitis
The incidence of ICI-related cholangitis is low, for example, 3.3–6.8% with Nivolumab [55], [56]. The median duration to the onset of cholangitis is approximately 11–22 weeks [56]. Abdominal pain is the most common symptom, followed by fever and jaundice [56]. ERCP-guided biopsy helps in establishing the diagnosis [56]. Corticosteroids are the mainstay of treatment but the response rate to steroid administration is low at about 11.5% [56].
CT shows variable intrahepatic biliary duct dilatation with diffusely thickened and enhancing walls of the bile duct (Fig. 11) [55]. MRI also shows intrahepatic biliary ductal dilatation with enhancement of the bile duct walls on post contrast imaging [57]. Pruned tree appearance of the bile ducts is seen in advanced cases as the condition progresses from extrahepatic bile ducts to intrahepatic bile ducts [57].
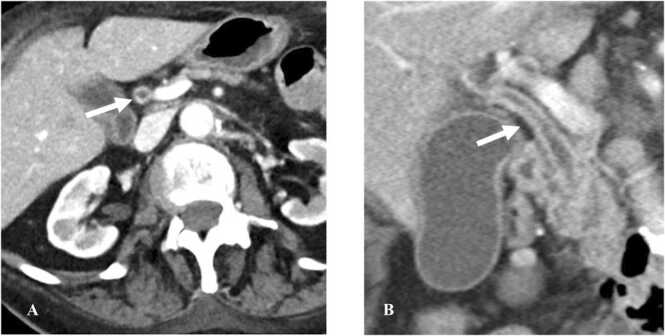
Fig. 11.
Immune-mediated cholangitis in a 72-year-old woman with melanoma on Pembrolizumab presenting with jaundice and elevated liver enzymes. A. Contrast-enhanced axial abdomen CT image shows enhancement in the wall of common bile duct (CBD) (arrow). B. Contrast-enhanced coronal abdomen CT image shows diffuse enhancement of the wall of CBD (arrow). Findings are consistent with cholangitis.
5.4. Cholecystitis
The incidence of ICI-related cholecystitis is low at 0.6% [58]. The median time to onset of cholecystitis is 6 months (0.1–31 months) [58]. Common symptoms are abdominal pain, nausea, diarrhea, and fever [58]. Approximately 20% of cases resolve with steroid administration while 80% cases are treated with percutaneous drainage or cholecystectomy [58].
On ultrasound, the gallbladder is distended with wall thickening, pericholecystic fluid and fat stranding. On CT, the gallbladder is distended with mucosal enhancement, enhancement of the adjacent liver parenchyma, gall bladder wall thickening with pericholecystic fluid and stranding (Fig. 12). PET/CT may show increased FDG uptake of the gallbladder wall.
Fig. 12.
Immune-mediated cholecystitis in an 88-year-old woman with squamous cell cancer of the left tonsil on pembrolizumab. A. Contrast-enhanced axial CT abdomen image shows distended gallbladder with enhancing mucosa and pericholecystic fluid (white arrow) suspicious for cholecystitis. B. Axial PET/CT image shows uptake in the gallbladder wall (black arrow). C. Fused axial PET/CT image shows FDG uptake in the gallbladder wall suggesting cholecystitis (dashed white arrow).
6. Endocrine irAE
6.1. Hypophysitis
The incidence of hypophysitis is higher with CTLA-4 inhibitors compared to PD-1/PD-L1 inhibitors [59]. A meta-analysis by Filette et al. reported that the incidence of hypophysitis with CTLA-4 inhibitors is 5.6%, PD-1/PD-L1 inhibitors is 0.5%, and combination therapy with CTLA-4 inhibitor and PD-1 inhibitor is 10.5% [59]. The disproportionate higher incidence of hypophysitis with CTLA-4 inhibitors is best explained by the presence of CTLA-4 granules in normal pituitary glands of some individuals [60]. The CTLA-4 inhibitors bind to the CTLA-4 granules and elicit type II and type IV immune responses causing T-cell lymphocytic infiltration of the pituitary which ultimately results in destruction of the gland [60], [61]. The median time to the onset of hypophysitis after starting immunotherapy is 2–3 months [62]. It is characterized by the onset of new symptoms of hypopituitarism after starting immunotherapy in the absence of alternate triggers or etiologies. Hypopituitarism presents with symptoms of endocrine dysfunction such as hypercortisolism (91%), hypothyroidism (84%), and hypogonadism (83%) [63]. Red flags include headache, loss of vision, and hypotension [63]. Hypophysitis is treated by cessation of ICI therapy and administration of steroids.
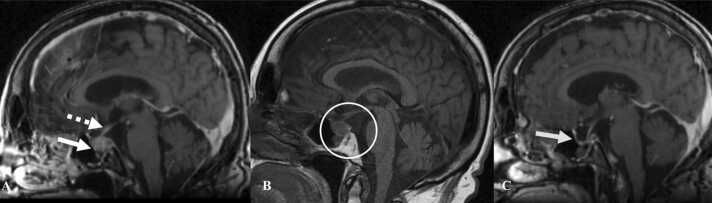
MRI is the best imaging modality to diagnose hypophysitis. There is diffuse enlargement of the pituitary gland and its stalk with loss of signal intensity in the posterior pituitary on pre-contrast sequences and homogenous enhancement on post-contrast sequences (Fig. 13) [64]. Geographic hypoenhancing lesions are noted in the anterior lobe due to fibrosis of the gland [65]. On PET/CT there is an initial increase in the FDG-uptake of the pituitary gland with subsequent decline in the uptake as the gland undergoes fibrosis [66].
Fig. 13.
Immune-mediated hypophysitis in a 70-year-old woman with melanoma on Pembrolizumab. A. Contrast-enhanced sagittal MRI image of the brain shows enlargement of the pituitary gland with convex superior margin (arrow) and thickened stalk (dashed white arrow). B. T1W sagittal MRI image of the brain shows absent posterior pituitary bright spot (white circle). C. Contrast-enhanced sagittal MRI image of the brain obtained after Pembrolizumab was stopped and steroid was administered shows significant decrease in the size of pituitary gland with empty sella turcica (arrow).
6.2. Thyroiditis
The incidence rate of thyroiditis with ICI therapy is 6–20% [1], [67]. Contrary to hypophysitis, the incidence of thyroiditis is higher with PD-1/PD-L1 inhibitors and lower with CTLA-4 inhibitors [68]. The median time to onset of thyrotoxic phase after initiating ICI is 5 weeks and hypothyroid phase is 10 weeks [68]. Thyroid dysfunction occurs in a biphasic pattern with an initial thyrotoxic phase followed by hypothyroidism due destruction of the gland. A prior study by Iyer et al. revealed that combined therapy has a higher incidence rate and shorter time to the onset of thyroiditis (2 weeks) [68]. Thyrotoxicosis is characterized by low thyroid stimulating hormone (TSH) and elevated functional T4 (fT4) while hypothyroidism is characterized by an elevated or normal TSH with low fT4 [68]. It is best treated with steroids and thyroid supplementation [68]. Beta blockers are reserved for symptomatic thyrotoxicosis.
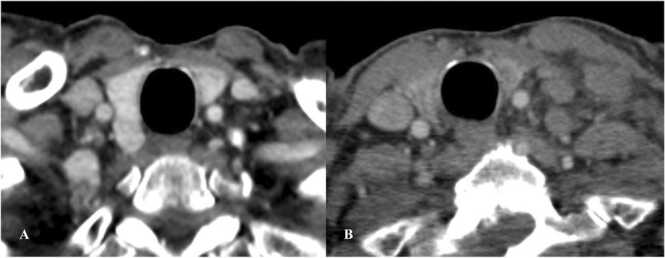
On thyroid ultrasound, the gland is heterogenous with low vascularity consistent with thyroiditis [68]. On CT, there is atrophy of the thyroid with decreased enhancement of the gland (Fig. 14) [68]. On PET/CT there is diffuse increase in FDG uptake initially followed by decrease in the uptake with development of fibrosis [68].
Fig. 14.
Immune-mediated thyroiditis in a 65-year-old man with lung cancer on Nivolumab. Contrast-enhanced axial chest CT images show A. Normal appearance of the thyroid before starting Nivolumab. B. There is atrophy of the thyroid with decrease in the enhancement after starting Nivolumab consistent with thyroiditis.
6.3. Adrenalitis
The incidence of adrenalitis ranges from 0.8-1.6% [40], [69]. The incidence rate is as high as 4–8% when combined CTLA-4 and PD-1/PD-L1 therapy is used [40], [69]. The median time to onset is 2.3–4.5 months [40], [69]. Primary adrenal insufficiency can be identified by electrolyte disturbances and eosinophilia with low serum cortisol along with high adrenocorticotrophin hormone (ACTH) [70]. Adrenal crisis, a life-threatening emergency, is rarely encountered with ICI therapy [1]. When adrenal crisis occurs, there is hypovolemic shock with nausea, vomiting, confusion, or coma [70]. Mild adrenal insufficiency is monitored and ICI therapy is continued [70]. Adrenal crisis is treated with intravenous steroids and fluid replacement [70].
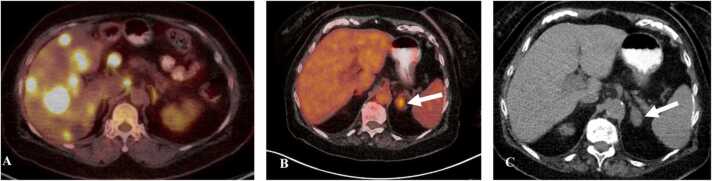
On CT, adrenalitis is characterized by smooth enlargement of the adrenal glands with homogenous enhancement on post contrast images (Fig. 15) [70]. On PET/CT there can be diffuse FDG uptake in the adrenal glands [70].
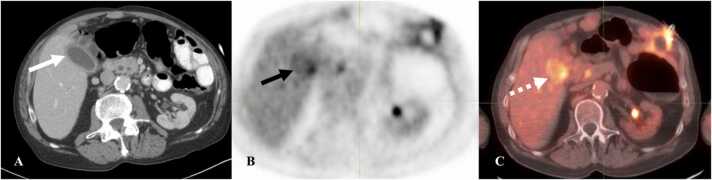
Fig. 15.
Immune-mediated adrenalitis in a 55-year-old man with melanoma on Pembrolizumab. A. Fused axial PET/CT image shows multiple hepatic metastases and mild FDG avidity in the left adrenal gland. B. Fused axial PET/CT image obtained after 6 months of initiation of Pembrolizumab therapy shows resolution of the hepatic metastases but new FDG uptake in the left adrenal gland (arrow). C. CT correlation shows new smooth enlargement of the left adrenal gland (arrow) seen with adrenalitis.
7. Musculoskeletal irAE
7.1. Inflammatory arthritis
The incidence rate of inflammatory arthritis is 5–12% in patients who received ICI therapy [71], [72]. The incidence rate is higher with combined therapy [71]. Median time to onset of arthritis is 3–4 months [72]. Prior studies have revealed that arthralgia or pain in the joints continues to persist after cessation of ICI therapy for 9 months or longer [73]. Symptomatic relief can be obtained by using non-steroidal anti-inflammatory drugs (NSAIDs) or low dose steroids and ICI therapy can be continued [72]. Some patients with more severe symptoms may require cessation of immunotherapy with high dose steroids and disease modifying anti-rheumatoid drugs (DMARDs) [72].
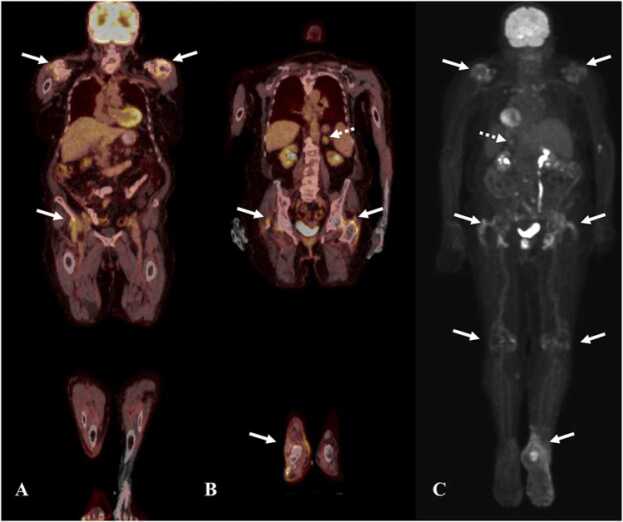
Synovitis is best identified on MRI. There is thickening, edema, and hyperenhancement of the synovium with joint effusion [72]. Increased periarticular FDG uptake is seen in synovial tissues on PET/CT [72]. Large joints are more commonly involved in symmetrical fashion [72]. Multiple joints are more involved with the median number of joints involved being four. The most commonly involved joints include the shoulders, knees, ankles, and wrists [72]. On PET/CT there is FDG avidity in the involved joints (Fig. 16).
Fig. 16.
Immune-mediated inflammatory arthritis and adrenalitis in a 63-year-old man with melanoma on Pembrolizumab therapy. A & B. Fused coronal PET/CT images show FDG uptake in bilateral shoulder joints (white arrows), bilateral hip joints and right ankle joint (white arrows), and left adrenal gland (dashed white arrow), C. Reconstructed MIP image shows uptake in bilateral shoulder joints, hip joints, right ankle joint (arrows) and left adrenal gland (dashed white arrow).
8. CNS irAE
Neurological irAEs are a rare complication of immunotherapy but can result in long-term morbidity. The incidence of neurological irAEs range from 3.8-12% [74]. The incidence is higher when combination therapy is used [74]. A wide-range of neurological manifestations such as myasthenia gravis, Guillain Barre’ syndrome, transverse myelitis, chronic inflammatory demyelinating polyneuropathy, meningitis, posterior reversible encephalopathy syndrome, and encephalopathy can be seen with immunotherapy related toxicity [75].
8.1. Toxic encephalopathy
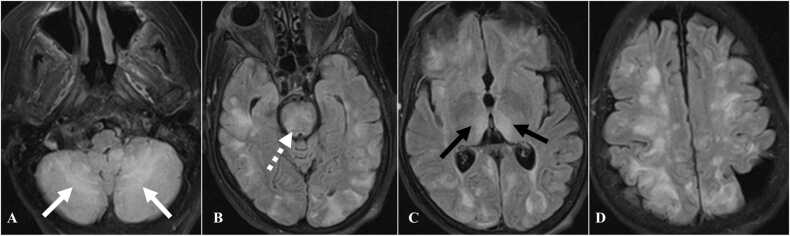
Patients who develop toxic encephalopathy due to immunotherapy can present with confusion, headache, vomiting, disorientation, and confabulation. CT may not show any findings in acute phase. MRI is the imaging modality of choice. On fluid-attenuation inversion recovery (FLAIR) sequence there is hyperintensity in subcortical and periventricular white matter, thalamus, corpus callosum, pons, and cerebellum (Fig. 17) [76].
Fig. 17.
Immune mediated toxic encephalopathy in a 69-year-old woman with lung cancer on Pembrolizumab therapy. A to D. Axial FLAIR images of MRI of the brain demonstrate subcortical and periventricular hyperintensities. Also seen are hyperintense areas in the cerebellum (white arrow), pons (dashed white arrow), and thalamus (black arrows).
9. Multiorgan irAEs
Multiorgan irAEs occur less frequently and range from 5.4-9.3% [77]. Patients with multiorgan irAEs have better response and improved survival compared to patients with single irAE. Prior studies have revealed that patients with multiorgan irAEs have 42% response rate and 53% reduced likelihood of death [78].
10. Conclusion
ICI therapy brought a paradigm shift in the treatment of solid and hematological malignancies. Though ICIs improve patient outcomes, they can be associated with adverse events in the form of irAEs. Patients who develop irAEs have better outcomes and improved survival than patients who do not develop irAEs. Therefore, it is vital to identify irAEs early and start mitigation measures immediately to continue beneficial ICI therapy without interruption. Radiologists must be aware of the imaging features of irAEs and alert the oncology team as soon as they identify a complication, albeit good prognostication, of ICI therapy.
Funding
None.
CRediT authorship contribution statement
Babina Gosangi: Conceptualization, Figures, Writing – original draft, Writing – review & editing. Lacey McIntosh: Conceptualization, Figures, Writing – review & editing. Abhishek Keraliya: Figures, Writing – review & editing. David V.K. Irugu: Figures, Writing – review & editing. Akshay Baheti: Writing – review & editing. Ashish Khandelwal: Writing – review & editing. Richard Thomas: Writing – review & editing. Marta Braschi Amirfarzan: Conceptualization, Figures, Writing – review & editing.
Conflict of Interest
None.
Contributor Information
Babina Gosangi, Email: babina.gosangi@yale.edu.
Lacey McIntosh, Email: Lacey.Mcintosh@umassmemorial.org.
Abhishek Keraliya, Email: akeraliya@bwh.harvard.edu.
David Victor Kumar Irugu, Email: drdvki2776@gmail.com.
Akshay Baheti, Email: akshaybaheti@gmail.com.
Ashish Khandelwal, Email: Khandelwal.Ashish@mayo.edu.
Richard Thomas, Email: Richard.Thomas1@lahey.org.
Marta Braschi-Amirfarzan, Email: Marta.Braschiamirfarzan@lahey.org.
References
- 1.Hodi F.S., O’Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 3.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.https://www.accessdata.fda.gov/scripts/cder/daf/ Accessed on 12/17/2021.
- 5.Khoja L., Day D., Wei-Wu Chen T., Siu L.L., Hansen A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann. Oncol. 2017;28(10):2377–2385. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 6.Toi Y., Sugawara S., Kawashima Y., Aiba T., et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncologist. 2018;23(11):1358–1365. doi: 10.1634/theoncologist.2017-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haratani K., Hayashi H., Chiba Y., et al. Association of immune-related adverse events with nivolumab efficacy in non–small-cell lung cancer. JAMA Oncol. 2018;4(3):374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf Accessed 12/17/2021
- 9.M. Nishino, H. Hatabu, F.S. Hodi, N.H. Ramaiya, Drug-related pneumonitis in the era of precision cancer therapy, JCO Precis Oncol. (2017) 1:PO.17.00026. Published 2017 May 26. doi:10.1200/PO.17.00026. [DOI] [PMC free article] [PubMed]
- 10.Naidoo J., Wang X., Woo K., et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J. Clin. Oncol. 2016;35:709–719. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishino M., Giobbe-Hurder A., Hatabu H. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2:1607–1616. doi: 10.1001/jamaoncol.2016.2453. [DOI] [PubMed] [Google Scholar]
- 12.Erasmus J.J., McAdams H.P., Rossi S.E. High-resolution CT of drug-induced lung disease. Radiol. Clin. N. Am. 2002;40:61–72. doi: 10.1016/s0033-8389(03)00109-x. [DOI] [PubMed] [Google Scholar]
- 13.Nishino M., Ramaiya N.H., Awad M.M., et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin. Cancer Res. 2016;22:6051–6060. doi: 10.1158/1078-0432.CCR-16-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishino M., Sholl L.M., Hodi F.S., et al. Anti-PD-1-related pneumonitis during cancer immunotherapy. N. Engl. J. Med. 2015;373:288–290. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishino M., Chambers E.S., Chong C.R., et al. Anti-PD-1 inhibitor-related pneumonitis in non–small-cell lung cancer. Cancer Immunol. Res. 2016;4:289–293. doi: 10.1158/2326-6066.CIR-15-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gkiozos I., Kopitopoulou A., Kalkanis A., Vamvakaris I.N., Judson M.A., Syrigos K.N. Sarcoidosis-like reactions induced by checkpoint inhibitors. J. Thorac. Oncol. 2018;13(8):1076–1082. doi: 10.1016/j.jtho.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Thomas S.N., Rohner N.A., Edwards E.E. Implications of lymphatic transport to lymph nodes in immunity and immunotherapy. Annu. Rev. Biomed. Eng. 2016;18:207–233. doi: 10.1146/annurev-bioeng-101515-014413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tirumani S.H., Ramaiya N.H., Keraliya A., Bailey N.D., Ott P.A., Hodi F.S., Nishino M. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol. Res. 2015;3(10):1185–1192. doi: 10.1158/2326-6066.CIR-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen R., Nørgaard P., Al-Jailawi M.K., Svane I.M. Late development of splenic sarcoidosis-like lesions in a patient with metastatic melanoma and long-lasting clinical response to ipilimumab. Oncoimmunology. 2014;3(8) doi: 10.4161/21624011.2014.954506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izzedine H., Gueutin V., Gharbi C., Mateus C., Robert C., Routier E., Thomas M., Baumelou A., Rouvier P. Kidney injuries related to ipilimumab. Invest New Drugs. 2014;32(4):769–773. doi: 10.1007/s10637-014-0092-7. [DOI] [PubMed] [Google Scholar]
- 21.Murphy K.P., Kennedy M.P., Barry J.E., O’Regan K.N., Power D.G. New-onset mediastinal and central nervous system sarcoidosis in a patient with metastatic melanoma undergoing CTLA4 monoclonal antibody treatment. Oncol. Res. Treat. 2014;37(6):351–353. doi: 10.1159/000362614. [DOI] [PubMed] [Google Scholar]
- 22.Teng F., Li M., Yu J. Radiation recall pneumonitis induced by PD-1/PD-L1 blockades: mechanisms and therapeutic implications. BMC Med. 2020;18(1):275. doi: 10.1186/s12916-020-01718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Huang Z., Xing L., Meng X., Yu J. Radiation recall pneumonitis induced by anti-PD-1 blockade: a case report and review of the literature. Front. Oncol. 2020;10:561. doi: 10.3389/fonc.2020.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibaki R., Akamatsu H., Fujimoto M., Koh Y., Yamamoto N. Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann. Oncol. 2017;28(6):1404–1405. doi: 10.1093/annonc/mdx115. [DOI] [PubMed] [Google Scholar]
- 25.Bradley J., Movsas B. Radiation pneumonitis and esophagitis in thoracic irradiation. Cancer Treat. Res. 2006;128:43–64. doi: 10.1007/0-387-25354-8_4. [DOI] [PubMed] [Google Scholar]
- 26.Wang H., Liao Z., Zhuang Y., Xu T., Nguyen Q.N., Levy L.B., O’Reilly M., Gold K.A., Gomez D.R. Do angiotensin-converting enzyme inhibitors reduce the risk of symptomatic radiation pneumonitis in patients with non-small cell lung cancer after definitive radiation therapy? Analysis of a single-institution database. Int. J. Radiat. Oncol. Biol. Phys. 2013;87(5):1071–1077. doi: 10.1016/j.ijrobp.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson D.B., Balko J.M., Compton M.L., et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmood S.S., Fradley M.G., Cohen J.V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moslehi J.J., Salem J.E., Sosman J.A., et al. Increased reporting of fatal immune checkpoint inhibitor‐associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salem J.E., Manouchehri A., Moey M., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonaca Marc P., Olenchock Benjamin A., et al. Myocarditis in the setting of cancer therapeutics. Circulation. 2019;140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aretz H.T. Myocarditis: the Dallas criteria. Hum. Pathol. 1987;18:619–624. doi: 10.1016/s0046-8177(87)80363-5. [DOI] [PubMed] [Google Scholar]
- 33.Palaskas N., Lopez-Mattei J., Durand J.B., et al. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J. Am. Heart Assoc. 2020;9(2) doi: 10.1161/JAHA.119.013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altan M., Toki M.I., Gettinger S.N., Carvajal-Hausdorf D.E., Zugazagoitia J., Sinard J.H., Herbst R.S., Rimm D.L. Immune checkpoint inhibitor-associated pericarditis. J. Thorac. Oncol. 2019;14(6):1102–1108. doi: 10.1016/j.jtho.2019.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.OPDIVO [package insert] Bristol-Myers Squibb Company; Princeton, NJ, 2017.
- 36.Daxini A., Cronin K., Sreih A.G. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin. Rheumatol. 2018;37(9):2579–2584. doi: 10.1007/s10067-018-4177-0. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe R., Zhang H., Berry G., Goronzy J.J., Weyand C.M. Immune checkpoint dysfunction in large and medium vessel vasculitis. Am. J. Physiol. Heart Circ. Physiol. 2017;312(5):H1052–H1059. doi: 10.1152/ajpheart.00024.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolaymat O.A., Pinkston O., Wang B., et al. Drug-associated vasculitis occurring after treatment with pembrolizumab. Rheumatology. 2019;58(Issue 8):1501–1503. doi: 10.1093/rheumatology/kez051. [DOI] [PubMed] [Google Scholar]
- 39.Kang A., Yuen M., Lee D.J. Nivolumab-induced systemic vasculitis. JAAD Case Rep. 2018;4(6):606–608. doi: 10.1016/j.jdcr.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibrahim R.A., Berman D.M., DePril V., Humphrey R.W., Chen T., Messina M., et al. Ipilimumab safety profile: Summary of findings from completed trials in advanced melanoma. J. Clin. Oncol. 2011;29 doi: 10.1200/jco.2011.29.15_suppl.8583. 8583 2915suppl. [DOI] [Google Scholar]
- 42.Kim K.W., Ramaiya N.H., Krajewski K.M., Shinagare A.B., Howard S.A., Jagannathan J.P., Ibrahim N. Ipilimumab-associated colitis: CT findings. AJR Am. J. Roentgenol. 2013;200(5):W468–W474. doi: 10.2214/AJR.12.9751. [DOI] [PubMed] [Google Scholar]
- 43.Thompson J.A., Schneider B.J., Brahmer J., et al. Management of immunotherapy-related toxicities, version 1.2019. J. Natl. Compr. Cancer Netw. 2019;17:255–289. doi: 10.6004/jnccn.2019.0013. [DOI] [PubMed] [Google Scholar]
- 44.Puzanov I., Diab A., Abdallah K., et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu J., Firpi-Morell R.J., Dang L.H., et al. An unusual case of gastritis in one patient receiving PD-1 blocking therapy: coexisting immune-related gastritis and cytomegaloviral infection. Gastroenterol. Res. 2018;11:383–387. doi: 10.14740/gr1068w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishimura Y., Yasuda M., Ocho K., Iwamuro M., Yamasaki O., Tanaka T., Otsuka F. Severe gastritis after administration of nivolumab and ipilimumab. Case Rep. Oncol. 2018;11(2):549–556. doi: 10.1159/000491862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayama N., Ihara H., Honma Y., Itoigawa Y., Kaira K., Fujii M. Severe gastritis due to pembrolizumab treatment in a lung cancer patient. Respirol. Case Rep. 2020:8. doi: 10.1002/rcr2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Messmer M., Upreti S., Tarabishy Y., et al. Ipilimumab-induced enteritis without colitis: a new challenge. Case Rep. Oncol. 2016;9(3):705–713. doi: 10.1159/000452403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larkin J., Chiarion‐Sileni V., Gonzalez R., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim K.W., Ramaiya N.H., Krajewski K.M., et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Investig. New Drugs. 2013;31:1071–1077. doi: 10.1007/s10637-013-9939-6. [DOI] [PubMed] [Google Scholar]
- 51.Brahmer J.R., Lacchetti C., Schneider B.J., et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2018 doi: 10.1200/JOP.18.00005. [DOI] [PubMed] [Google Scholar]
- 52.Horvat T.Z., Adel N.G., Dang T.O., et al. Immune‐related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abu-Sbeih H., Tang T., Lu Y., Thirumurthi S., Altan M., Jazaeri A.A., Dadu R., Coronel E., Wang Y. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J. Immunother. Cancer. 2019;7(1):31. doi: 10.1186/s40425-019-0502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das J.P., Postow M.A., Friedman C.F., Do R.K., Halpenny D.F. Imaging findings of immune checkpoint inhibitor associated pancreatitis. Eur. J. Radiol. 2020;131 doi: 10.1016/j.ejrad.2020.109250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawakami H., Tanizaki J., Tanaka K., et al. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Investig. New Drugs. 2017;35(4):529–536. doi: 10.1007/s10637-017-0453-0. [DOI] [PubMed] [Google Scholar]
- 56.Onoyama T., Takeda Y., Yamashita T., et al. Programmed cell death-1 inhibitor-related sclerosing cholangitis: a systematic review. World J. Gastroenterol. 2020;26(3):353–365. doi: 10.3748/wjg.v26.i3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takinami Masaki, Ono Akira, Moriguchi Michihisa, Takahashi Toshiaki. Pembrolizumab-related sclerosing cholangitis manifested by a pruned-tree appearance of the bile ducts. Jpn. J. Clin. Oncol. 2021;51(Issue 4):666–667. doi: 10.1093/jjco/hyaa201. [DOI] [PubMed] [Google Scholar]
- 58.Abu-Sbeih H., Tran C.N., Ge P.S., Bhutani M.S., Alasadi M., Naing A., Jazaeri A.A., Wang Y. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J. Immunother. Cancer. 2019;7(1):118. doi: 10.1186/s40425-019-0604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Filette J., Andreescu C., Cools F., Bravenboer B., Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm. Metab. Res. 2019;51(03):145–156. doi: 10.1055/a-0843-3366. [DOI] [PubMed] [Google Scholar]
- 60.Gubbi S., Hannah-Shmouni F., Verbalis J.G., Koch C.A. Hypophysitis: an update on the novel forms, diagnosis and management of disorders of pituitary inflammation. Best. Pract. Res. Clin. Endocrinol. Metab. 2019;33 doi: 10.1016/j.beem.2019.101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caturegli P., Di Dalmazi G., Lombardi M., et al. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am. J. Pathol. 2016;186:3225–3235. doi: 10.1016/j.ajpath.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gedye C., van der Westhuizen A., John T. Checkpoint immunotherapy for cancer: superior survival, unaccustomed toxicities. Intern. Med. J. 2015;45(7):696–701. doi: 10.1111/imj.12653. [DOI] [PubMed] [Google Scholar]
- 63.Caturegli P., Di Dalmazi G., Lombardi M., et al. Hmiypophysitis secondary to cytotoxic T-lymphocyte–associated protein 4 blockade. Am. J. Pathol. 2016;186(12):3225–3235. doi: 10.1016/j.ajpath.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faje A., Reynolds K., Zubiri L., Lawrence D., Cohen J.V., Sullivan R.J., Nachtigall L., Tritos N. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur. J. Endocrinol. 2019;181(3):211–219. doi: 10.1530/EJE-19-0238. [DOI] [PubMed] [Google Scholar]
- 65.Kurokawa R., Ota Y., Gonoi W., et al. MRI findings of immune checkpoint inhibitor-induced hypophysitis: possible association with fibrosis. AJNR Am. J. Neuroradiol. 2020;41(9):1683–1689. doi: 10.3174/ajnr.A6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lasocki A., Iravani A., Galligan A. The imaging of immunotherapy-related hypophysitis and other pituitary lesions in oncology patients. Clin. Radiol. 2021;76(5):325–332. doi: 10.1016/j.crad.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 67.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., et al. CheckMate 067: a Phase III randomized double-blind study of nivolumab (NIVO) monotherapy or NIVO combined with ipilimumab (IPI) versus IPI monotherapy in previously untreated patients (pts) with advanced melanoma (MEL) Ann. Oncol. 2015;26 28–28. [Google Scholar]
- 68.Iyer P.C., Cabanillas M.E., Waguespack S.G., et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018 doi: 10.1089/thy.2018.0116. ahead of print. Available at: www.liebertpub.com/doi/abs/10.1089/thy.2018.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atkins M.B., Choueiri T.K., Hodi F.S., et al. Pembrolizumab (MK-3475) plus low-dose ipilimumab (IPI) in patients (pts) with advanced melanoma (MEL) or renal cell carcinoma (RCC): data from the KEYNOTE-029 phase 1 study. J. Clin. Oncol. 2015;33(15 Suppl):3009. doi: 10.1200/jco.2015.33.15_suppl.3009). [DOI] [Google Scholar]
- 70.Cukier P., Santini F.C., Scaranti M., Hoff A.O. Endocrine side effects of cancer immunotherapy. Endocr. Relat. Cancer. 2017;24(12):T331–T347. doi: 10.1530/ERC-17-0358. [DOI] [PubMed] [Google Scholar]
- 71.Cappelli L.C., Gutierrez A.K., Bingham C.O., et al. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res. 2017;69:1751–1763. doi: 10.1002/acr.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buder-Bakhaya K., Benesova K., Schulz C., et al. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol. Immunother. 2018;67:175–182. doi: 10.1007/s00262-017-2069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith M.H., Bass A.R. Arthritis after cancer immunotherapy: symptom duration and treatment response. Arthritis Care Res. 2019;71(3):362–366. doi: 10.1002/acr.23467. [DOI] [PubMed] [Google Scholar]
- 74.Cuzzubbo S., Javeri F., Tissier M., Roumi A., Barlog C., Doridam J., Lebbe C., Belin C., Ursu R., Carpentier A.F. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur. J. Cancer. 2017;73:1–8. doi: 10.1016/j.ejca.2016.12.001. Epub 2017 Jan 5. PMID: 28064139. [DOI] [PubMed] [Google Scholar]
- 75.Feng S., Coward J., McCaffrey E., Coucher J., Kalokerinos P., O’Byrne K. Pembrolizumab-induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J. Thorac. Oncol. 2017;12(11):1626–1635. doi: 10.1016/j.jtho.2017.08.007. Epub 2017 Aug 24. PMID: 28843363. [DOI] [PubMed] [Google Scholar]
- 76.Khoja L., Maurice C., Chappell M., et al. Eosinophilic fasciitis and acute encephalopathy toxicity from pembrolizumab treatment of a patient with metastatic melanoma. Cancer Immunol. Res. 2016;4(3):175–178. doi: 10.1158/2326-6066.CIR-15-0186. Epub 2016 Jan 28. PMID: 26822024. [DOI] [PubMed] [Google Scholar]
- 77.Kichenadasse G., Miners J.O., Mangoni A.A., Rowland A., Hopkins A.M., Sorich M.J. Multiorgan immune-related adverse events during treatment with atezolizumab. J. Natl. Compr. Cancer Netw. 2020;18(9):1191–1199. doi: 10.6004/jnccn.2020.7567. [DOI] [PubMed] [Google Scholar]
- 78.Zhou X., Yao Z., Yang H., et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020;18:87. doi: 10.1186/s12916-020-01549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]