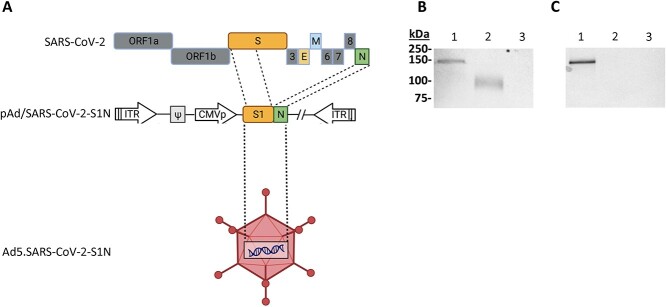
Figure 1.
Adenoviral-vectored SARS-CoV-2-S1N vaccine. (A) A shuttle vector carrying the codon-optimized SARS-CoV-2-S1 gene encoding N-terminal 1–661 along with full Nucleoprotein was designed as shown in the diagram. The vector was used to generate recombinant type 5 replication-deficient adenoviruses (Ad5) by homologous recombination with the adenoviral genomic DNA, shown by the BioRender illustration of Ad5.SARS-CoV-2-S1N. ITR, inverted terminal repeat. (B) Detection of the SARS-CoV-2-S1N fusion protein by western blot with the supernatant of A549 cells infected with Ad5.SARS-CoV-2.S1N (Ad5.S1N) using S1 SARS-CoV-2 rabbit polyclonal antibody (lane 1). As a positive control, supernatant of A549 cells infected with Ad5.SARS-CoV-2.S1 (Ad5.S1) was loaded (lane 2). As a negative control, supernatant of A549 cells infected with an empty vector (AdΨ5) was loaded (lane 3). (C) Detection of the SARS-CoV-2-S1N fusion protein by western blot with the supernatant of A549 cells infected with Ad5.SARS-CoV-2.S1N (Ad5.S1N) using N SARS-CoV-2 rabbit polyclonal antibody (lane 1). As a negative control, supernatant of A549 cells infected with Ad5.SARS-CoV-2.S1 (Ad5.S1) was loaded (lane 2). As a negative control, supernatant of A549 cells infected with an empty vector (AdΨ5) was also loaded (lane 3). The supernatants were resolved on SDS-10% polyacrylamide gel after being boiled in 2% SDS sample buffer with β-ME.