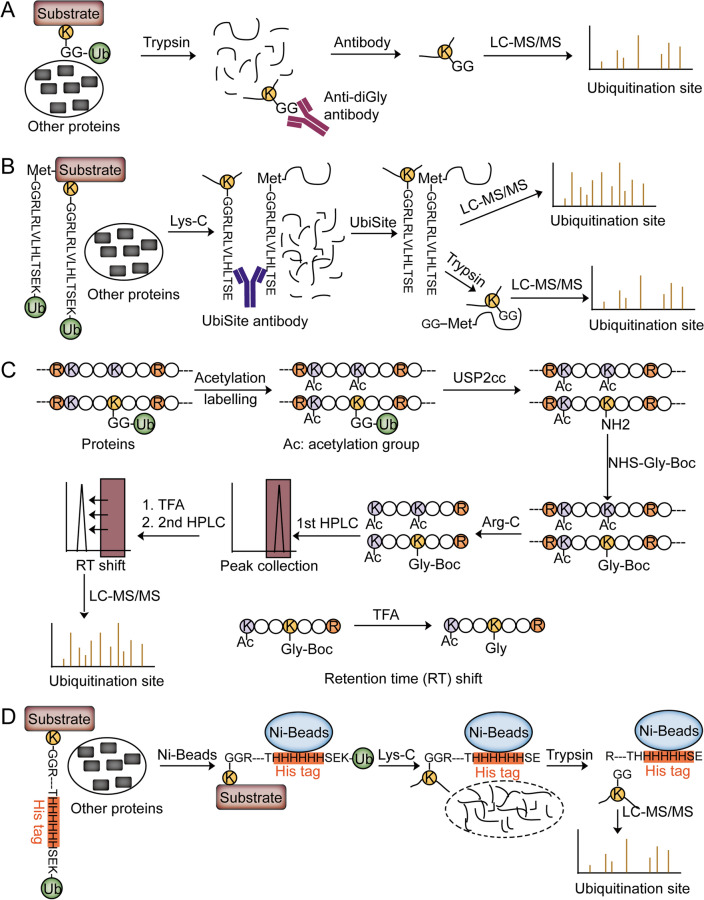
Fig. 3.
Approaches to identifying ubiquitination sites followed by the enrichment of ubiquitinated peptides. A Schematic workflow of anti-diGly antibody-based approach. The whole cell lysates are directly digested with trypsin. Then, the diGly-modified peptides are further enriched by the anti-diGly antibody for LC–MS/MS analysis. B Schematic workflow of UbiSite antibody-based approach. Ubiquitinated proteins are first digested with Lys-C. Then, the ubiquitinated peptides containing the ESTLHLVLRLRGG sequence are enriched by the UbiSite antibody. The enriched peptides are directly analyzed or analyzed after tryptic digestion by LC–MS/MS. C Schematic workflow of Ub-COFRADIC approach. After labeling the lysine ε-amine by acetylation, the ubiquitin moieties are hydrolyzed from the substrates using USP2cc to reveal a free primary amine on the ubiquitinated lysine. After Gly-Boc labeling and Arg-C digestion, the peptides are separated by a first RP-HPLC into some fractions. Each fraction is acidized by TFA to remove the Boc group. The retention time of Gly-Boc-modified peptides is different from the retention time of Gly-modified peptides. After secondary RP-HPLC runs, the peaks with a retention time shift are collected and analyzed by LC–MS/MS, resulting in ubiquitination site identification. D Schematic workflow of StUbEx PLUS approach. Ubiquitinated proteins are purified by Ni-beads and digested with Lys-C to specifically cleave after Lys residues (K). Therefore, the non-ubiquitinated peptides are released from the Ni-beads and the ubiquitinated peptides are still attached to the Ni-beads. By specifically cleaving after Arg residues (R), the ubiquitinated peptides containing diGly remnants are purified from the Ni-beads and identified by LC–MS/MS analysis