Table 2.
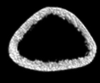
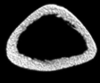
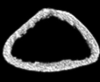
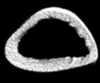
Results of cortical bone morphometrical analysis for the nonoperated side
| Sham | C | R | VD | R + VD | |
|---|---|---|---|---|---|

|
|
|
|
|
|
| BV of cortical bone (mm3) | 4.65 ± 0.46 | 4.87 ± 0.25 | 5.05 ± 0.26 | 4.65 ± 0.34 | 5.15 ± 0.39 |
| Cr. Ar (mm2) | 5.68 ± 0.56 | 5.96 ± 0.30 | 6.18 ± 0.32 | 5.69 ± 0.42 | 6.29 ± 0.48 |
| Cr. Th (mm) | 0.36 ± 0.04 | 0.36 ± 0.02 | 0.39 ± 0.03 | 0.34 ± 0.03 | 0.38 ± 0.04 |
Sham, sham group; C, control group; R, romosozumab group; VD, active vitamin D3 group; and R + VD, romosozumab + active vitamin D3 group. Cr. Ar, cortical bone area; Cr. Th, cortical bone thickness. All parameters showed a greater increase in the R and R + VD groups than in the C group, but no significant differences were observed in BV, area, and thickness of the cortical bone between the groups
