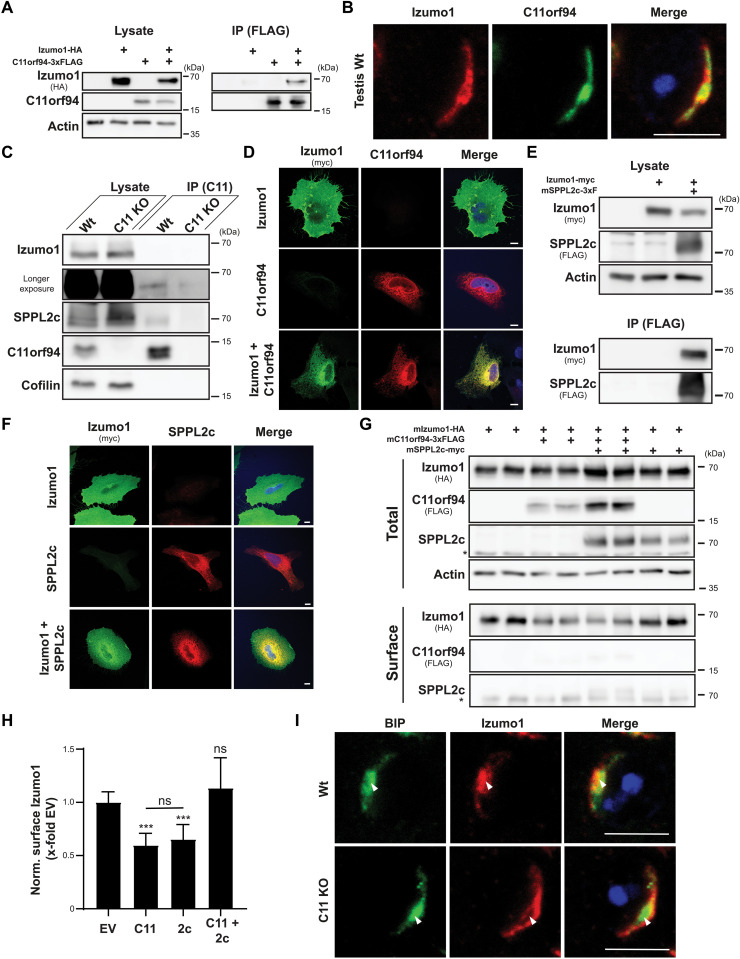
Fig. 5. C11orf94 regulates ER exit of Izumo1.
(A) CHAPSO-solubilized proteins from transfected HeLa cells were precipitated using anti-FLAG beads. Total lysates and bead eluates (IP) were analyzed by Western blotting. (B) Testis cryosections from Wt mice were stained with anti-Izumo1, anti-C11orf94, and DAPI. Scale bar, 5 μm. (C) C11orf94 was precipitated from CHAPSO homogenates from Wt or C11orf94−/− (C11 KO) testes using anti-C11orf94 Sepharose. Lysates and eluates were analyzed by Western blotting. (D) Transfected HeLa cells were fixed and subjected to indirect immunofluorescence analysis. Scale bars, 10 μm. (E) Interaction of Izumo1-myc and SPPL2c-3xFLAG was analyzed as described in (A). (F) Subcellular localization of SPPL2c-3xFLAG and Izumo1-myc was visualized by indirect immunofluorescence in transfected HeLa cells. Scale bars, 10 μm. (G) After biotinylation of surface proteins, transfected HeLa cells were lysed and biotinylated proteins were precipitated using streptavidin-coated beads. Lysates and bead eluates (IP) were subjected to Western blot analysis. Asterisk, unspecific band. (H) Quantification of (G). N = 3 to 6, n = 6 to 12, one-way ANOVA with Tukey’s post hoc test. Statistical significance compared to EV-transfected control is indicated above the bars. (I) Colocalization of Izumo1 with the ER marker protein BIP in Wt or C11 KO round spermatids was analyzed by IHC. ***P ≤ 0.001. Scale bars, 5μm.