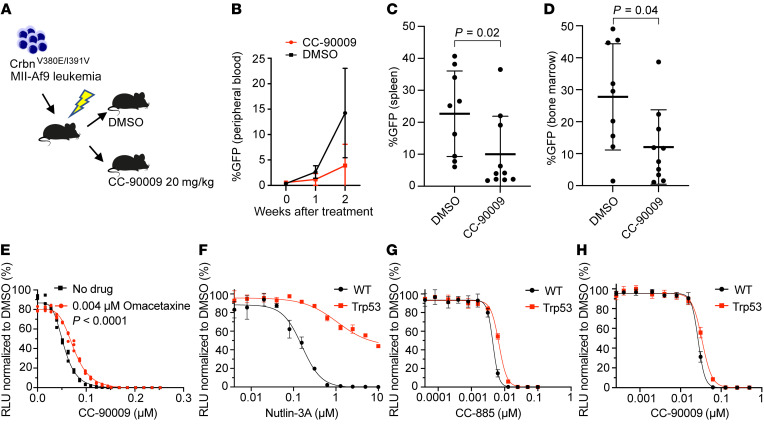
Figure 7. In vivo treatment with CC-90009 can treat CrbnV380E/I391V leukemia.
(A) Design of secondary transplant experiment. (B) Assessment of GFP+ (Mll-Af9) cells in peripheral blood in DMSO-treated and CC-90009–treated (20 mg/kg) mice before treatment and at 1 week and 2 weeks after treatment. (C and D) Assessment of disease burden (GFP+ cells) in spleen (C) and bone marrow (D) 2 weeks after treatment with either DMSO or CC-90009 (20 mg/kg). (E) Cell viability in Mll-Af9 CrbnV380E/I391V cells treated with 0.004 mM omacetaxine in combination with CC-90009 at the indicated doses. Cell viability was assessed using CellTiter-Glo luminescent assay 72 hours after treatment (shown is a representative experiment performed in technical triplicate). (F–H) Cell viability in Mll-Af9 CrbnV380E/I391V cells in a TP53-WT or TP53-null (Trp53) background as indicated following treatment with nutlin (F), CC-885 (G), and CC-90009 (H). Cell viability was assessed using CellTiter-Glo luminescent assay 72 hours after treatment (shown are representative experiments performed in technical triplicate).