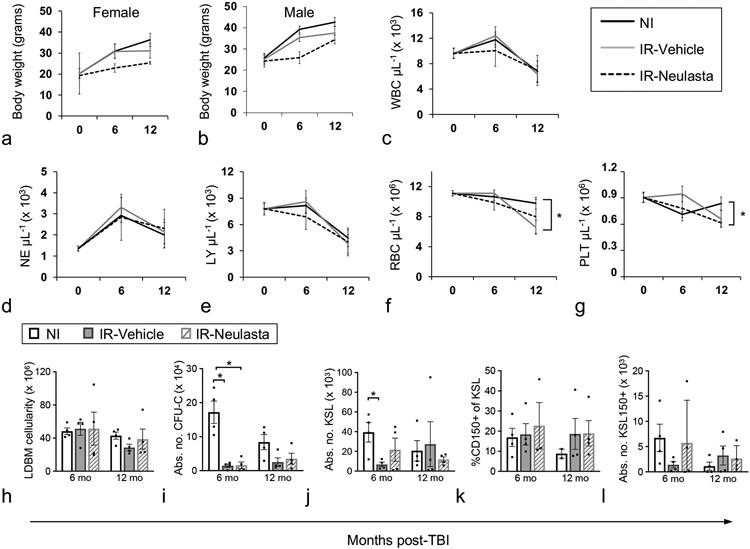
Figure 5.
Body weights, CBC, and primitive hematopoietic BM cells of vehicle- and Neulasta-treated JDO mice 6 and 12 months after lethal TBI. Male and female JDO mice were exposed to 9.0 Gy, injected subcutaneously with 2 doses of 1 mg kg−1 Neulasta or vehicle at 24-26h and d8 post-irradiation, euthanized at 6 and 12 mo post-TBI and assessed for female (a) and male (b) body weights, and total PB WBC (c), NE (d), LY (e), RBC (f), and PLT (g). n = 20–80 mice for baseline values (0, age=2 months), n = 4–17 mice per group for 6 and 12 mo post-TBI time points. BM cells were assessed for total number of mononuclear cells per mouse (h), total colony forming unit cells per mouse (CFU-C, i), number of KSL cells per mouse (KSL, j), CD150+ cells as a percentage of KSL (k), and number of KSL150+ cells per mouse (l). NI mice were analyzed non-contemporaneously with Vehicle and Neulasta mice. n = 4 mice per group except NI CD150 analysis at 12 mo where n=2. Data are mean ± SEM; *p<0.05 comparing groups at each time point.