Fig. 1. G protein-coupled receptor signaling.
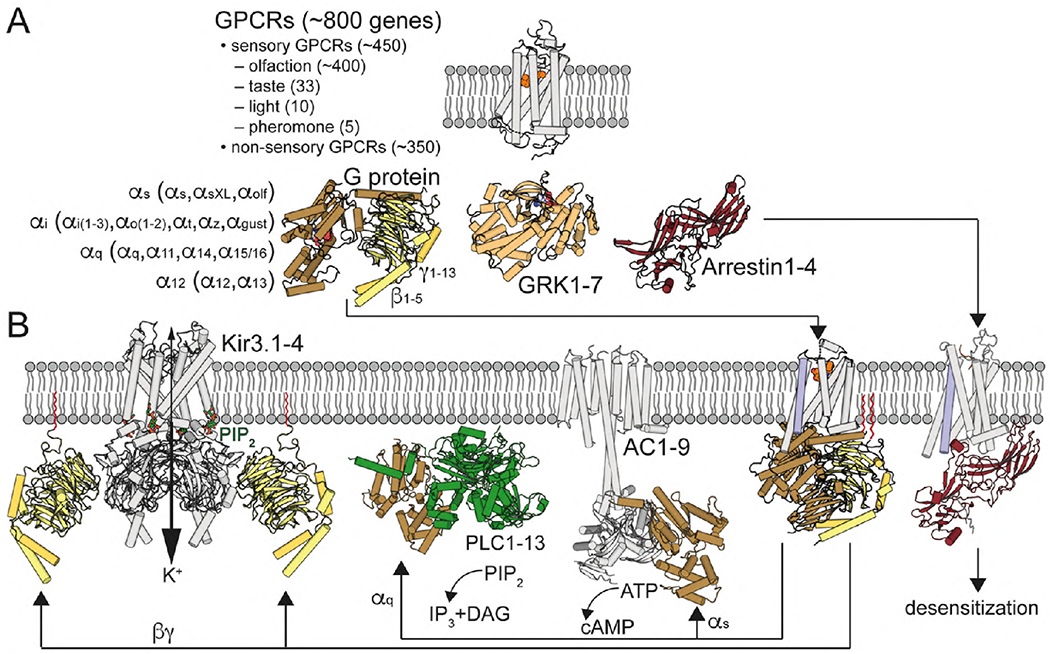
(A) GPCRs are illustrated with the structure of retinal-bound rhodopsin in white (Palczewski et al., 2000). In humans, there are 16 Gα subunits (classified as Gαs, Gαi, Gαq and Gα12) 5 Gβ and 12 Gγ (Gγ1-13, but no Gγ6) subunits. Active GPCRs interact with Gαβγ proteins, illustrated with the structure of GDP-bound Gαi1β1γ2 in brown/yellow (Wall et al., 1995), G protein-coupled receptor kinases (GRKs), with 7 mammalian isoforms, illustrated with the structure of ATP-bound GRK1 in light orange (Singh, Wang, Maeda et al., 2008), and arrestins, with 4 isoforms, illustrated with the structure of arrestin1 in red (Granzin et al., 1998). (B) Agonist binding triggers the movement of TM6 (in blue), opening an intracellular cavity for G protein arrangement, illustrated with the crystal structure of the agonist-β2AR-Gs complex (Rasmussen et al., 2011). Gαq activates PLC, with 13 mammalian isozymes, illustrated with the crystal structure of PLC-β3 bound to Gαq (Waldo et al., 2010), which catalyzes the hydrolysis of PIP2 to the Ca2+-mobilizing second messenger inositol 1,4,5-trisphosphate (IP3) and the PKC-activating second messenger diacylglycerol (DAG). Gαs stimulates and Gαi inhibits the activity of AC, with 9 mammalian isoforms, illustrated with the cryo-EM structure of AC9 bound to Gαs (Qi et al., 2019). Gβγ subunits increase the activity of Kir3 channels, illustrated with the crystal structure of the Kir3.2-Gβγ (Whorton and MacKinnon, 2011). Desensitization is mediated by GRK-mediated GPCR phosphorylation and subsequent binding of arrestin, illustrated with the cryo-EM structure of neurotensin NT1 receptor in complex with arrestin2 (Huang et al., 2020). Gγ isoprenylated group is shown in red.
