Fig. 4. Allosterism in GPCR heteromers.
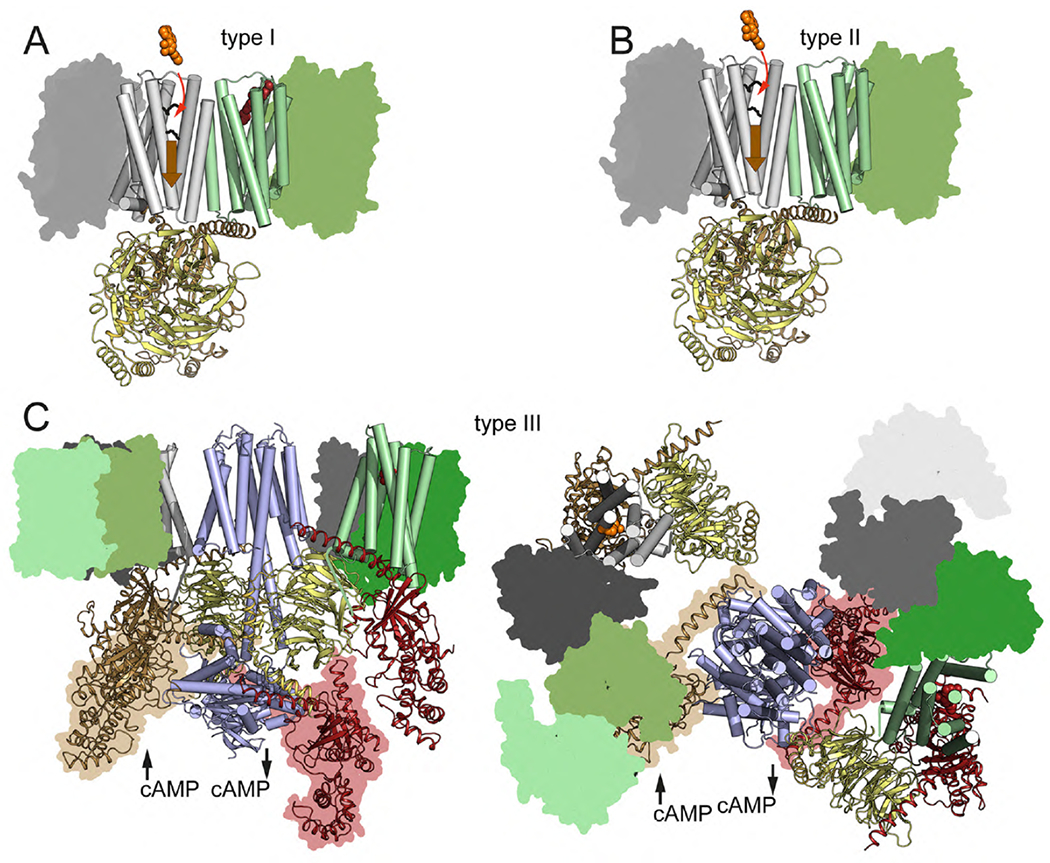
(A) Type I allosterism, in which a ligand (red spheres) binding to one protomer (green cylinders) in the GPCR heteromer can modify the affinity (ligand binding depicted by the red arrow) or the efficacy (receptor activation depicted by the wide orange arrow) of another ligand (orange spheres) binding to the partner receptor (gray cylinders) via their TM helices (see Fig. 5). (B) Type II allosterism, or ligand-independent allosterism, in which the affinity or efficacy of a ligand (orange spheres) for a GPCR (gray cylinders) can be modified just by heteromerization with a molecularly different GPCR (green cylinders). (C) Lateral view (left) and extracellular view (right) of a computer model of a GEMMA including two heterotetramers composed of two different GPCR homomers (one represented by white cylinders and grey surfaces and the other represented by green cylinders and surfaces), AC (light blue cylinders; based on the cryo-EM structure of AC9; Qi et al, 2019), Gs (brown for Gαs and yellow for Gβγ) and Gi (red for Gαi and yellow for Gβγ) (based on Navarro et al., 2018). This type of GEMMA can explain the ability of a Gi-coupled GPCR to counteract AC activation mediated by a Gs-coupled GPCR (type III allosterism). The proposed contact between the CT domain of the receptor and the Gβ subunit is shown by a color line (Tsai et al. 2019). To facilitate visualization of all protomers of the GPCR oligomers, one of the protomers is represented by cylinders and the other protomers are represented as color surfaces (with different shades of the same color for the same GPCR). Both positions of the Gαs and Gαi subunits, in their Gβγ-associated and receptor-bound and state (without surface) and in their Gβγ-dissociated and AC-bound state (with surface), are shown. The agonist-induced dissociation of Gαs and Gαi from their respective Gβγ takes place within the framework of the GEMMA..
