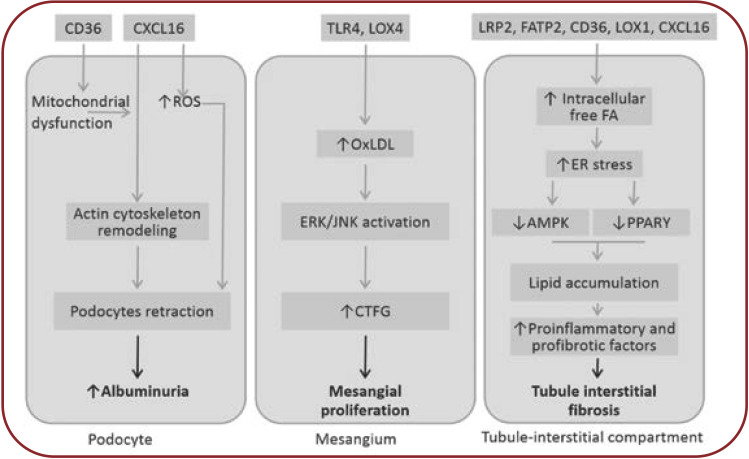
FIGURE 2.
Potential effects of hyperlipemia on kidney (18-23). AMPK=AMP activated protein kinase; CTGF=conective tissue growth factor; ER=endoplasmic reticulum; ERK=extracelular signal-regulated kinase; JNK=c-jun-NH2 terminal kinase; PPARá=peroxisome proliferator-activated receptor alpha; ROS=reactive oxygen species. Ox-LDL are taken over by “scavenger” receptors (SR-A, CD 36, CXCL-16) resulting in foam cells. Scavenger receptor CXCL-16 mediates podocytes uptake of ox-LDL, which induces loss of nephrin expression, contributing to proteinuria and glomerulosclerosis. Nephrin loss also affects insulin-stimulated glucose uptake in podocytes, which will exacerbate the endoplasmic reticulum stress initiated by podocyte lipid accumulation. Unlike other glomerular structures, the mesangial cells, being not covered by the basement membrane, will more easily meet Ox-LDL, which are taken by special “scavenger” receptors like TLR 4 (toll-like receptors) or LOX-1 (lectin-like oxidized LDL receptor). Thereafter, TGF-â/Smad signaling and PAI-1 transcription (plasminogen activator inhibitor-1) are activated, leading to progression of kidney failure. Overexpression of SREBP-1 and fatty acid synthase leads to increase in renal triglyceride contents. These alterations increase TGFâ1 and vascular endothelial growth factor expression, which contribute to increased mesangial matrix expression, glomerulosclerosis and proteinuria. Tubule-interstitial involvement is mainly due to albumin transported fatty acids and, to a lesser extent, to direct action of both native and oxidized LDL forms. Following high lipid exposure, tubular epithelial cells change their phenotype to a pro-inflammatory one, with increased permeability, chemotactic stimulation and leukocyte adherence. Cellular permeability is affected by reduced E-cadherin expression and FAK overexpression. Tubular cells exhibit ICAM-1, whose expression is MAPK-dependent, which promotes neutrophiles trafficking along the peritubular capillaries. These alterations in tubular cells may play a crucial role in the development and progression of tubular dysfunction.