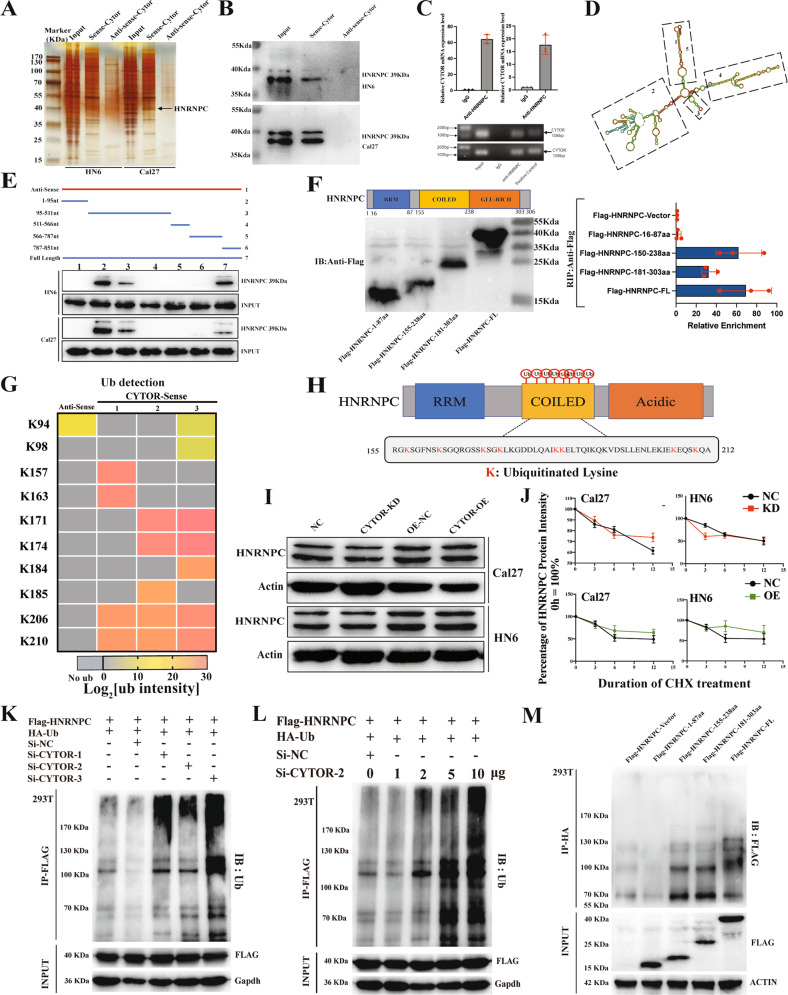
Fig. 3. CYTOR inhibits the non-degradative ubiquitination of HNRNPC.
A Sense and antisense of CYTOR were transcribed, biotinylated in vitro, and incubated with protein extracts from HN6 and Cal27 cells for RNA pull-down assays. The pulled-down proteins were used for silver staining, and a specific band appeared at ~39 kDa (black arrow). B Immunoblotting for specific interactions of HNRNPC with CYTOR in HN6 and Cal27 cells. C RIP assays were performed using antibody against HNRNPC and normal rabbit IgG. Median values are plotted in the column with data generated from three technical replicates, statistical analysis was performed by unpaired t-tests. D Graphic illustration of the predicted secondary structure of CYTOR using RNA-fold software. E Immunoblotting for HNRNPC in samples pulled down with full-length CYTOR, truncated CYTOR ((1) 1–95 nt; (2) 95–511 nt; (3) 511–566 nt; (4) 566–787 nt; and (5) 787–851 nt) or the antisense of CYTOR. Upper panel: graphic illustration of truncated CYTOR probe according to the secondary structure. Lower panel: immunoblotting for HNRNPC in protein samples pulled down by the different truncated mutants of CYTOR. F HNRNPC was truncated (1-87 aa, 155-238 aa, and 181-303 aa) according to its protein domains to identify the specific domain of HNRNPC which interacts with CYTOR. RIP assays were performed to detect the enrichment of CYTOR in cells transfected with full-length and truncated Flag-tagged constructs. Left panel: immunoblotting for truncations of Flag-tagged recombinant HNRNPC protein. Right panel: the enrichment of CYTOR in different truncations were measured by qPCR. Data were generated from three technical replicates. G Heatmap of ubiquitinated lysine residues within HNRNPC with normalized log2 abundance of CYTOR pull-down samples. Colors correspond to the intensity of detected ubiquitination by mass spectrometry. H Eight ubiquitinated lysine residues (K157, K163, K171, K174, K184, K185, K206, K210) which located within the coiled-coil domain of HNRNPC were detected. I Immunoblotting for HNRNPC protein expression in OSCC cells. J CYTOR-overexpressing and CYTOR-knockdown HN6 and Cal27 cells were treated with cycloheximide (CHX, 50 μg/mL) for the indicated times. K, L 293 T cells were transfected with indicated plasmids or small interferon-RNAs and followed by immunoprecipitation (IP), the immunoblotting for HA-Ub showed the ubiquitination of HNRNPC protein. M Truncated HNRNPC plasmids were transfected into 293 T cells to evaluate the ubiquitination activity in specific domains. (KD knockdown, NC negative control, OE overexpression; The data was presented as mean ± SD).