Abstract
The burden of severe Covid-19 has been relatively low in sib-Saharan Africa compared to Europe and the Americas. However, SARS-CoV-2 sero-prevalence data has demonstrated that there has been more widespread transmission than can be deduced from reported cases. This could be attributed to under reporting due to low testing capacity or high numbers of asymptomatic SARS-CoV-2 infection in communities. Recent data indicates that prior SARS-CoV-2 exposure is protective against reinfection and that vaccination of previously SARS-CoV-2 infected individuals induces robust cross-reactive antibody responses. Considering these data, calls for a need for a re-think of the COVID-19 vaccination strategy in sub-Saharan African settings with high SARSCoV-2 population exposure but limited available vaccine doses. A potential recommendation would be to prioritize rapid and widespread vaccination of the first dose, while waiting for more vaccines to become available.
Keywords: SARS-CoV-2, Africa, burden, vaccines
The recorded burden of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) referred to as coronavirus disease 2019 (COVID-19) has been relatively low in sub-Saharan Africa (SSA) compared with Europe and the Americas. However, SARS-CoV-2 seroprevalence data suggests that case counts may be orders of magnitude higher than those reported because of low testing capacity and high prevalence of asymptomatic infections in the community, among other factors [4]. Sero surveillance studies have shown seroprevalence rates as high as 70% in several subpopulations, indicating widespread viral transmission even prior to the onset of circulation of Omicron variant [2–6]. Although the incidence of severe COVID-19 is relatively low in SSA, continued viral transmission in a context of low vaccine coverage creates room for the emergence of viral variants of concern. We highlight in this article some peculiarities of the ongoing pandemic on the African continent, which is home to 18% of the world’s population, and suggest options to tackle the current challenges while addressing future pandemic preparedness.
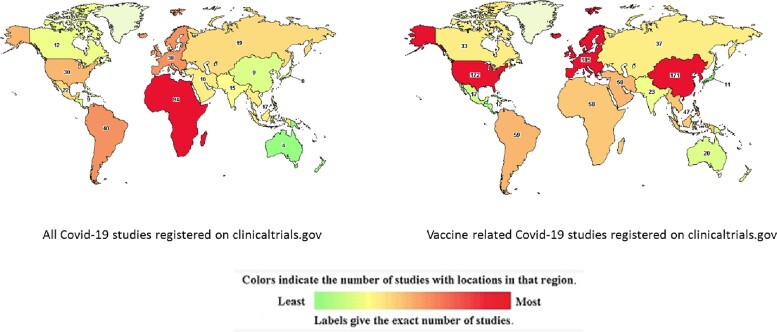
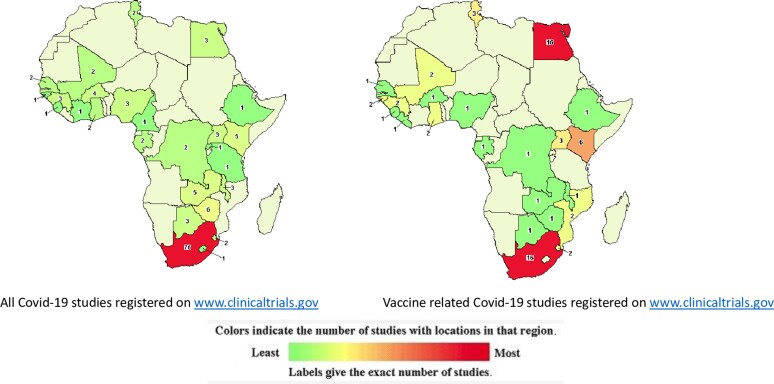
Between 2010 and 2016, 53 publications/million population were published on a range of vaccine-related topics in South Africa, 84/million in Guinea Bissau, and 127/million in The Gambia [7]. Despite having this historically rich vaccine research activity, less than 10% of all studies related to coronavirus disease 2019 (COVID-19) have been conducted on the African continent (Figures 1 and 2). Only 5% of all COVID vaccine trials were conducted on the continent that houses 18% of global population. Of more than 500 vaccine trials done globally, only 20 are in Africa; of these, only 2 are effectiveness studies [8, 9]. More than one-half of all COVID-19 studies registered on the continent are not vaccine trials but studies on COVID-19 more broadly, and typically smaller studies measuring immune responses. This dearth of COVID vaccine research effort on the continent may partially stem from the fact that public health and biomedical research in Africa has historically been driven by partnerships led by institutions in the global West. Within these partnerships, insufficient investment in local capacity development has limited the growth and autonomy of African scientists based in African institutions. This lack of empowerment has been exacerbated by inadequate domestic funding of research by African governments and foundations, which ensures that much of research agenda, governance, and oversight remains under the purview of the global West. Within the specialized field of immunology, vaccine science and vaccine development, few African and continent-based scientists had established an ongoing research capacity that could be pivoted quickly to tackle the emerging need for COVID vaccine research. Another crucial factor is probably that none of the vaccines in use today was manufactured on the continent. In the context of optimizing time to market, it would be practical to have early trials conducted in the local context where they were manufactured. Later phase trials are however necessary globally to tackle a global pandemic.
Figure 1.
Distribution of studies related to coronavirus disease 2019 worldwide. Image source https://www.clinicaltrials.gov downloaded 20 February 2022.
Figure 2.
Distribution of studies related to coronavirus disease 2019 in Africa. Image source https://www.clinicaltrials.gov downloaded 20 February 2022.
The only published work to date on effectiveness of COVID-19 vaccines in Africa, is from South Africa; the Astra Zeneca COVID-19 vaccine effectiveness trial with primary objective of effectiveness of vaccine 2 weeks after the second dose [10]. By the time participants in this trial reached this primary objective time point, the Beta variant had begun to dominate in the region. A total of 85% of healthy participants unsurprisingly did not show neutralizing antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the vaccine was not efficacious in protecting against mild to moderate COVID-19 [10]. The World Health Organization (WHO) recommended continued use of the vaccine because there was good evidence that vaccine could still protect against severe disease [11]. Subsequently, it was demonstrated in Canada that even a single dose of the Astra Zeneca vaccine was associated with reduced risk of severe disease from the Beta or Gamma variant. A single dose of Johnson and Johnson vaccine trialed in several countries, including South Africa, in individuals with underlying comorbidities, protected against 64% of moderate to severe disease 28 days after vaccination and reduced severe COVID-19 from the Beta variant by 82% 28 days after vaccination [12]. A single dose also reduced the risk of healthcare workers being hospitalized by 65% and of death by 92% in this group. This was higher if 2 doses were received [12]. These local data are useful to guide local vaccination policy in addition to the global body of data.
Vaccine Availability and Rollout on the Continent
In October 2021, the WHO and other United Nations bodies called for international commitment and support to ensure that every country will have at least 40% of its population vaccinated with at least 1 dose of a COVID vaccine by the end of 2021 [13]. No African country achieved or came close to achieving 40% population vaccination coverage by the end of 2021.
Several countries have lamented the short shelf life of the vaccines delivered to them. As was the case during the H1N1 pandemic in 2010 in which one-third of 32.3 million vaccine doses delivered to 34 African countries were wasted, a similar trajectory is playing out with SARS-CoV-2 [14]. Significant distribution bottlenecks have accounted in part for the huge equity gap in vaccine access demonstrated by recent data. By September 30, 2021, the proportion of the population fully vaccinated against SARS-CoV-2 in the United States was 54% compared with 0.2% in Benin [15]. Although there are several contributors to this disparity, as detailed in the following section, the disparity in vaccine access is a problem for the countries that have access to vaccine as well as those that do not. Continued transmission of the virus, in unvaccinated populations creates room for viral mutations to emerge as result of undervaccinated population and some of these mutations or variants can escape current vaccines in use and pose a threat to all including the vaccinated population.
There are several possible reasons for the problem with vaccine access in Africa and other low- and middle-income countries (Figure 3).
Figure 3.
Why access to coronavirus disease 2019 vaccines is a problem in Africa and other low- and middle-income countries.
Production Capacity or Production Gaps
Globally, there is insufficient production capacity. As of March 2021, there were only about 7 countries that were producing COVID-19 vaccines [16]. Estimates showed that with the available capacity then, 5 billion doses would be produced annually; however, the world needs more than 7 billion doses annually to combat the COVID-19 disease. Another challenge is the constraint in the supply of raw materials. In addition, to secure global access, it is crucial that the governments remove their objections to intellectual property waivers and export restrictions, and actively facilitate the production of vaccines in places where they are needed.
Procurement
To help with vaccine procurement for low- and middle-income countries, COVAX, headed by Gavi the Vaccine Alliance and the Coalition for Epidemic Preparedness and Innovations was established with the aim of delivering 2 billion doses of vaccines to low- and middle-income countries and other countries that have subscribed to the facility by the end of 2021. The principle is to ensure fair and equitable access to COVID-19 vaccines for every country around the world, with target of ensuring that at least 20% of the population in countries that have subscribed are covered by the vaccines. Because of production delays and import and export restriction from India following the devastating effect of the Delta variant, COVAX has not been unable to deliver on this promise, leaving Africa still challenged.
In addition, the limited financial power of low- and middle-income countries to sign bilateral deals with vaccine producers is a hindrance. The proportion of the vaccine doses procured by direct use and direct deals should normally be paid for by national budgets. For low-income countries, only 6% of the doses are expected to be procured from direct deals, so financial constraints of the countries in West Africa and Africa limit these countries from buying vaccines from the limited supply. Low- and middle-income countries are unable to compete with the rich countries for the limited supply of vaccine and they are dependent on COVAX and the African Vaccination Trust initiative to provide vaccines for them. The important and significant contributions of these organizations however only cover a fraction of the potential need.
Distribution Bottleneck
The United States, European Union, and other rich countries who have excess vaccine doses indicated an intention to donate this excess to low- and middle-income countries, but most of these had not been delivered by November 2021. The United States, for example, delivered 841 million doses to Africa. But there were a further 191 million doses for delivery by the end of 2021. This is a result of the slow speed of the donation of excess doses from high-income countries. On the other hand, African countries need to be ready to absorb the increase in supply of vaccines once the vaccines are delivered. Given the projections for vaccine doses over time, African countries need to be ready to absorb the increase in supply. This means that African countries need to prepare, the health system must be in place, and health workers trained and available. The social mobilization and communication advocacy to address vaccine hesitancy needs to be optimal and the financing made available for the delivery of the vaccines.
The solutions to COVID-19 vaccines access challenges and vaccine access more broadly lie in 3 major issues: (1) additional donation to COVAX so that it can continue to supply countries; (2) fast track of donations of excess doses from rich countries; and (3) the development of vaccine manufacturing capacity in Africa, and particularly the support of the Trade Related Aspects of Intellectual Property Rights waiver, to ensure that more companies in Africa and other middle-income countries are able to contribute to developing and producing these products to ease the bottleneck that we have experienced. Community engagement remains key within the context to facilitate roll out and uptake (Figure 4).
Figure 4.
Solutions to the coronavirus disease 2019 vaccine access challenges.
As a continent, there is need to take responsibility and ownership of the prevailing health challenges. Since the 2016 declaration by African ministers of health of need for vaccine manufacturing capacity on the continent, little progress was made until recently in the midst of the pandemic and the attendant paucity of vaccine doses for the continent when progress toward establishing this capacity restarted. The appropriate schedule for COVID-19 vaccine use on the continent also needs to be explored and this requires additional local data, as the schedules used in high-income countries may not be best suited to the continent. This is particularly so in the context that large portions of the populace (up to 70% in some settings) may have already been exposed to the virus. A single primary dose schedule may be more suitable at least until supplies increase. Because recent data indicate that prior SARS-CoV-2 exposure provides some protection against reinfection [17, 18], and that vaccination of previously SARS-CoV-2–infected individuals induces robust cross-reactive antibody responses [19, 20], a single-dose strategy in the context may be more suited. Local data-driven policies will likely play a major role in determining the most appropriate strategies for effect disease control on the continent. This strategy will best be driven with local investment.
To forestall future situations such as in the context of the COVID-19 pandemic, there needs to be investment by the public, private, and academic sectors to make the continent more competitive for research, development and manufacture of COVID-19 and other vaccines. Researchers on the continent need to define and follow through the most pressing research questions and implementations strategies adapting and innovating as relevant to the context. Although the current push for manufacturing COVID-19 vaccines on the continent is welcome, the push may best serve the need for the larger scale development of vaccines for which there is significant ongoing need. Natural infection with COVID-19 may obviate some of the urgency of the need for vaccination. Studies assessing neutralization activity across a panel of SARS-CoV-2 variants of concern/interest are warranted to estimate community immunity, the duration of this immunity in context, and thus guide local policy. Social science research addressing growing vaccine hesitancy and distribution bottlenecks also need to be addressed.
Notes
Financial support. This work was supported by the Precision Vaccine Programme.
Supplement sponsorship . This supplement is sponsored by the Precision Vaccines Program of Boston Children’s Hospital.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Olubukola T Idoko, Faculty of Infectious and Tropical disease, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Effua Usuf, Faculty of Infectious and Tropical disease, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Uduak Okomo, Faculty of Infectious and Tropical disease, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Chizoba Wonodi, International Health, Health Systems Center, John Hopkins University, Baltimore, USA.
Kondwani Jambo, Viral Immunology Research Group, Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Blantyre, Malawi.
Beate Kampmann, Faculty of Infectious and Tropical disease, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Shabir Madhi, South African Medical Research Council Vaccines and Infectious Diseases Analytics Research Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Ifedayo Adetifa, Faculty of Infectious and Tropical disease, London School of Hygiene and Tropical Medicine, London, United Kingdom.
References
- 1. Sagara I, Woodford J, Kone M, et al. . Rapidly increasing SARS-CoV-2 seroprevalence and limited clinical disease in three Malian communities: a prospective cohort study. medRxiv 2021. doi: 10.1101/2021.04.26.21256016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morton B, Barnes KG, Anscombe C, et al. . Distinct clinical and immunological profiles of patients with evidence of SARS-CoV-2 infection in sub-Saharan Africa. Nat Commun 2021; 12:3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salako AO, Amoo OS, Odubela OO, et al. . Prevalence and clinical characteristics of coronavirus disease 2019 seen at a testing sentre in Lagos Nigeria. West Afr J Med 2021; 38:54–8. [PubMed] [Google Scholar]
- 4. Mandolo J, Msefula J, Henrion MYR, et al. . SARS-CoV-2 exposure in Malawian blood donors: an analysis of seroprevalence and variant dynamics between January 2020 and July 2021. BMC Med 2021; 19:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uyoga S, Adetifa IMO, Karanja HK, et al. . Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science 2021; :371:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Razafimahatratra SL, Ndiaye MDB, Rasoloharimanana LT, et al. . Seroprevalence of ancestral and Beta SARS-CoV-2 antibodies in Malagasy blood donors. Lancet Glob Health 2021; 9:e1363–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moisi J, Madhi SA, Rees H. Vaccinology in sub-Saharan Africa. BMJ Glob Health 2019; 4:e001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shinde V, Bhikha S, Hoosain Z, et al. . Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021; 384:1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Madhi SA, Izu A, Pollard AJ. ChAdOx1 nCoV-19 vaccine efficacy against the B.1.351 variant. Reply. N Engl J Med 2021; 385:571–2. [DOI] [PubMed] [Google Scholar]
- 10. Madhi SA, Baillie V, Cutland CL, et al. . Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 Variant. N Engl J Med 2021; 384:1885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . Interim recommendations for use of the ChAdOx1-S [recombinant] vaccine against COVID-19 (AstraZeneca COVID-19 vaccine AZD1222 Vaxzevria™, SII COVISHIELD™).
- 12. Sadoff J, Gray G, Vandebosch A, et al. . Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO . Strategy to Achieve Global Covid-19 Vaccination by mid-2022.
- 14. Mihigo R, Torrealba CV, Coninx K, et al. . 2009 Pandemic influenza A virus subtype H1N1 vaccination in Africa–successes and challenges. J Infect Dis 2012; 206(Suppl 1):S22–8. [DOI] [PubMed] [Google Scholar]
- 15. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. . A global database of COVID-19 vaccinations. Nat Hum Behav 2021; 5:947–53. [DOI] [PubMed] [Google Scholar]
- 16. Lawler D. Where are Covid-19 vaccines coming from 2021. Available from: https://www.axios.com/covid-coronavirus-vaccines-astrazeneca-pfizer-357c2fa9-3df8-4115-9baa-c5b2375beb44.html.
- 17. Abu-Raddad LJ, Chemaitelly H, Coyle P, et al. . SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine 2021; 35:100861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hall VJ, Foulkes S, Charlett A, et al. . SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet 2021; 397:1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ebinger JE, Fert-Bober J, Printsev I, et al. . Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 2021; 27:981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stamatatos L, Czartoski J, Wan Y-H, et al. . mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021. doi: 10.1126/science.abg9175 [DOI] [PMC free article] [PubMed] [Google Scholar]