Abstract
Older adults, defined as those ≥60 years of age, are a growing population vulnerable to infections including severe acute respiratory syndrome coronavirus 2. Although immunization is a key to protecting this population, immunosenescence can impair responses to vaccines. Adjuvants can increase the immunogenicity of vaccine antigens but have not been systematically compared in older adults. We conducted a scoping review to assess the comparative effectiveness of adjuvants in aged populations. Adjuvants AS01, MF59, AS03, and CpG-oligodeoxynucleotide, included in licensed vaccines, are effective in older human adults. A growing menu of investigational adjuvants, such as Matrix-M and CpG plus alum, showed promising results in early phase clinical trials and preclinical studies. Most studies assessed only 1 or 2 adjuvants and no study has directly compared >3 adjuvants among older adults. Enhanced preclinical approaches enabling direct comparison of multiple adjuvants including human in vitro modeling and age-specific animal models may derisk and accelerate vaccine development for older adults.
Keywords: vaccines, adjuvants, older adults, infectious diseases, coronavirus
We conducted a scoping review to assess the efficacy of adjuvanted vaccines among older adults. There is a growing pipeline of adjuvant candidates under development that may be effective in this age group.
The size and proportion of older adults within the general population is growing across the globe. The global population aged ≥60 years is projected to rise from 962 million in 2017 to 2.1 billion by 2050 [1]. Older adults are vulnerable to multiple infectious diseases including viral respiratory infections such as influenza and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). For example, compared to young adults aged 18–29 years in the United States, older adults aged >85 years have a >600-fold mortality risk from coronavirus disease 2019 (COVID-19), the disease caused by SARS-CoV-2 [2]. Developing safe and effective vaccines for this vulnerable population is crucial. However, immunosenescence, an altered immune function in older adults, not only increases their risk and severity of infection but also impairs their response to immunization [3]. In this context, a precision medicine approach may help identify vaccine formulations tailored to this immunologically vulnerable population [4].
To develop vaccines with enhanced immunogenicity for older adults, approaches have focused on increasing the antigen dose, altering routes of administration (eg, intradermal), and adjuvanting existing vaccines, the latter of which is the focus of this scoping review. Adjuvants are components that can enhance antigen-specific humoral and/or cell-mediated immunity typically by activating receptors of the innate immune system called pattern recognition receptors (PRRs) and/or by modulating antigen pharmacokinetics [5, 6]. Alum is the most frequently used adjuvant in vaccine formulations approved for clinical use and was the only available adjuvant for decades. However, a range of new adjuvants have been developed over the past 20–30 years such as oil-in-water (O/W) emulsions and Toll-like receptor (TLR) agonists, and several new adjuvants have proven effective at enhancing immune responses in immunocompromised populations including older adults. For example, O/W emulsions MF59 and AS03 as well as AS01, a liposome-based adjuvant containing monophosphoryl lipid A (MPL) and the saponin QS-21, have recently been incorporated in licensed influenza and zoster vaccines for older adults [7]. Adjuvants boost the immune system and can: (1) decrease the amount of antigen contained in a vaccine (ie, antigen sparing); (2) reduce the number of vaccine doses required to achieve protection (ie, dose sparing); and/or (3) broaden the immune response to variable antigens, all of which are important for effective and scalable vaccine development. The urgent need for accelerated vaccine development prompted us to conduct a scoping review of adjuvanted vaccines targeting older adults, aiming to assess their efficacy, identify promising agents that enhance immunogenicity of vaccinal antigens, and highlight knowledge gaps. We found that several adjuvants, such as MF59, AS03, AS01, and CpG-oligodeoxynucleotides (CpG-ODN), demonstrate effectiveness in older adults. Despite a growing pipeline of adjuvants [8, 9], including those discovered via National Institutes of Health/National Institutes of Allergy and Infectious Disease’s adjuvant discovery program [10], comparative effectiveness human studies of 2 or more adjuvants are very limited in older adults, highlighting the importance of preclinical platforms enabling head-to-head comparisons of multiple adjuvants.
METHODS
Search Strategy
We conducted a scoping review to evaluate effects of vaccine adjuvants in older adults. Both clinical and preclinical (eg, human in vitro and animal model) studies were included. Adjuvants were defined as components of vaccines given in any dose, formulation, and route to enhance immunogenicity against infection. The literature search was conducted by a medical librarian on 31 March 2020 and updated on 24 September 2021, using PubMed, CINAHL, EMBASE, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov. The bibliographies of included studies were manually reviewed for additional references. EndNote and Covidence were used to manage citations. The search query was developed jointly by the librarian and physician authors, using combinations and variations of the terms, including “aged,” “elderly,” “vaccination,” “immunization,” “adjuvant,” “immunostimulant,” “immunoactivator,” and the names and chemical makeup of known adjuvants (Supplementary Table 1). There were no date limits applied to the search.
Eligibility Criteria
We included human studies of adults who were healthy or had stable underlying chronic medical conditions aged >60 years old, or 50 years old in the case of zoster vaccine studies. Populations in which >50% of the participants had concomitant diseases known to influence immune function (eg, diabetes, chronic kidney disease, posttransplantation) were excluded. Because the equivalence of older adult age differs between human and animal species, we included nonhuman studies describing the animals as “aged,” noting the age equivalence in the relevant results section. Studies including other adult age groups were eligible if the aged group was analyzed separately. The other inclusion criteria were English language and original research articles. Conference abstracts, commentaries, and letters were excluded. Review articles and meta-analyses were screened for original research articles of relevance but not included as primary data sources.
Screening, Data Extraction, and Synthesis
Two authors (E. N., A. A.) independently screened titles and abstracts and performed full-text assessments. Any discrepancies between them over the eligibility of studies were resolved through discussion or after consultation with a third team member (O. L.). Data extracted included information on adjuvant and vaccine, study population, study design and setting, and study findings. These were summarized across common parameters to generate graphical analyses and summary tables. Immunogenicity of adjuvanted vaccines in older adults was presented as a fold-change of the mean antibody (Ab) titer over the comparator (eg, nonadjuvanted vaccine in older adults, adjuvanted and/or nonadjuvanted vaccine in young adults) whenever available.
RESULTS
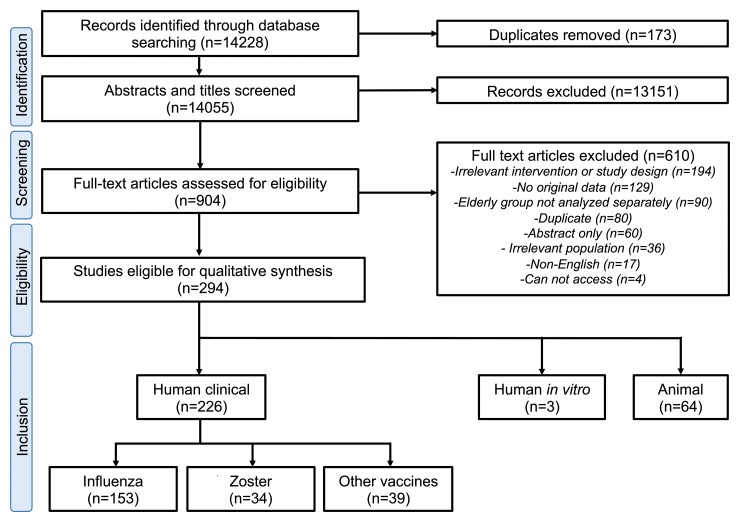
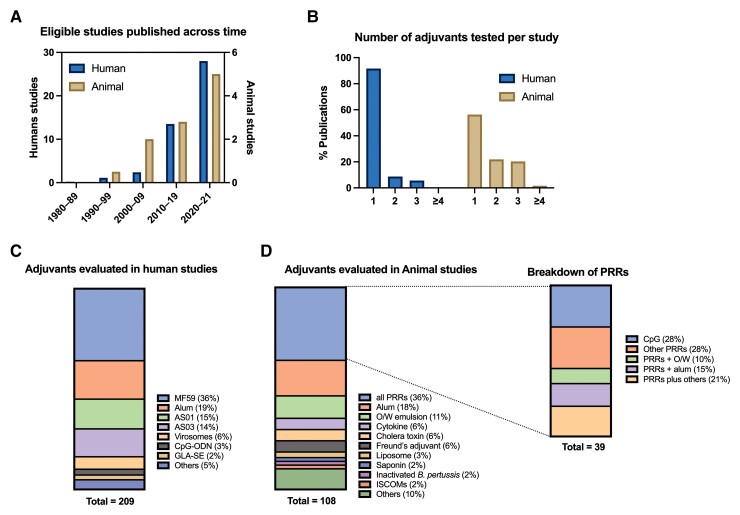
Our literature search yielded 14 055 records after deduplication, which were screened for title and abstract (Figure 1). After excluding 13 151 records, we retrieved 904 full-text articles for further eligibility screening. A total of 294 articles were eligible for inclusion. We noted an increasing number of publications in this field across time for both human and animal studies with variety of adjuvants (Figure 2A, C, and D). Each study was categorized either as human clinical, human in vitro, or animal study; a summary of eligible articles is provided in Supplementary Tables 2–4.
Figure 1.
Flow diagram of scoping review approach for adjuvants in older adults. Each phase of the process is outlined including identification, screening eligibility, and inclusion as well as number of articles in each step. The number of down selected records is outlined in brackets.
Figure 2.
Summary of selected articles as evaluated in animal and human studies. A, Numbers of eligible studies published over time. B, Numbers of adjuvant(s) studied per study. Breakdown of adjuvants evaluated in human (C) and animal (D) studies. Abbreviations: B. pertussis, Bordetella pertussis; GLA-SE, glucopyranosyl lipid A in stable oil-in-water emulsion; ISCOM, immunostimulating complex; O/W, oil-in-water; PRR, pattern recognition receptor.
Human Clinical Studies of Adjuvanted Herpes Zoster Vaccines
Our full-text screening captured 34 human studies focusing on the same adjuvanted recombinant herpes zoster subunit (HZ/su) vaccine. The HZ/su vaccine contains varicella-zoster virus glycoprotein E (gE) and adjuvant system AS01, which is a unique combination of immunostimulatory components—the TLR4 ligand MPL and the saponin fraction QS-21 in liposomes. The HZ/su vaccine has shown remarkable efficacy in human older adults in 2 pivotal randomized placebo-controlled trials [11, 12]. The first study, ZOE-50, included participants ≥50 years of age and demonstrated overall efficacy of 97.2% compared with placebo in reducing the risk of herpes zoster [12]. The second study, ZOE-70, was for participants >70 years of age and also demonstrated excellent efficacy of HZ/su (89.9%) in advanced age [11]. The majority of HZ/su recipients in all age groups showed robust Ab and cell-mediated immune responses against gE after vaccination [13]. A comparative immunogenicity study demonstrated that the HZ/su vaccine induces significantly higher varicella-zoster virus-specific interleukin-2+ (IL-2+) and gE-specific IL-2+ interferon-γ+ T cells than the live attenuated zoster vaccine [14]. The HZ/su vaccine was approved by the Food and Drug Administration for prevention of HZ in adults ≥50 years of age in 2017, and has since been the focus of multiple meta-analyses/systematic reviews representing most safety and efficacy studies from our screen. Of note, a systematic review of 27 studies with network meta-analysis demonstrated that HZ/su was statistically superior to both the conventional live attenuated vaccine and placebo, with efficacy of 85% and 94%, respectively, in adults ≥50 years of age [15]. Although the HZ/su vaccine was associated with more injection site reactions than the live attenuated vaccine, it was well tolerated and no causality was established with serious adverse events (AEs) [15].
Human Clinical Studies of Adjuvanted Influenza Vaccines
After full-text screening, we identified 153 eligible influenza vaccine studies. Among 138 studies specifying the adjuvants used, the O/W emulsion adjuvants MF59 (73 studies) and AS03 (30 studies) included in licensed influenza vaccines, were the most extensively studied, followed by alum (25 studies) and flagellin (3 studies). MF59-adjuvanted influenza vaccines in older adults have been evaluated in several meta-analyses and systematic reviews with regard to immunogenicity, efficacy/effectiveness, and safety. The largest systematic review and meta-analysis of 39 studies demonstrated that adults aged ≥60 years of age who received MF59-adjuvanted vaccine had significantly higher Ab titers compared with those who received a standard dose nonadjuvanted vaccine [16]. In a meta-analysis of 16 studies evaluating real-world vaccine effectiveness (VE) in adults ≥65Y, the MF59-adjuvanted vaccine demonstrated significant absolute VE and improved relative VE for the prevention of influenza-related medical encounters compared with nonadjuvanted trivalent (pooled estimate of +13.9%) and quadrivalent (pooled estimate of +13.7%) influenza vaccines [17]. AS03-adjuvanted influenza vaccine also induced robust Ab responses, whereas modest Ab responses were evident after receipt of alum-adjuvanted H5N1 influenza vaccine [18]. Several systematic reviews and meta-analyses have focused on safety in older adults, with the most comprehensive one demonstrating significantly increased risk of local AEs following MF59- and AS03-adjuvanted vaccines compared with control vaccines. AS03 was also associated with fatigue and myalgia; however, neither adjuvant was associated with serious AEs [19]. We did not find any human studies comparing influenza vaccine adjuvants head-to-head.
Human Clinical Studies of Other Vaccines
In addition to influenza and herpes zoster vaccines, our screening yielded 39 human clinical studies, largely randomized clinical trials, on adjuvanted vaccines in various stages of development (35% phase 1, 34% phase 2, 25% phase 3, and 6% phase 4). Average participant age was 70 years (range 50–102 years), and the median number of participants was 156 per study. Vaccines were given intramuscularly with 1 exception given intradermally. Thirty-one studies reported immunogenicity outcomes, mostly assessing humoral immunity with Ab concentrations/titers, with some also reporting seroconversion/seroprotection rates. Limited number of studies evaluated cell-mediated immunity (n = 7) and protection from the disease of interest (n = 5). In contrast to influenza and zoster vaccine studies, there was a wide variety of adjuvants and vaccinal antigens studied. The top 4 vaccinal antigens studied were SARS-CoV-2 (n = 8), respiratory syncytial virus (RSV) (n = 6), hepatitis B (n = 6), and pneumococcus (n = 6). Alum was the most studied adjuvant, followed by TLR9 agonist CpG-ODN and TLR4 agonist glucopyranosyl lipid A in stable O/W emulsion (GLA-SE). Each study tested 1–3 adjuvants at 1–3 doses (Figure 2B). Among these various adjuvant-antigen combinations, the most effective adjuvant in older adults appeared to be the CpG-adjuvanted hepatitis B vaccine. A 2-dose regimen of this vaccine, composed of recombinant hepatitis B surface antigen and the TLR9 agonist CpG 1018, generated higher seroprotection levels in older adults compared to a 3-dose regimen of the conventional alum-adjuvanted hepatitis B vaccine and was approved by the Food and Drug Administration in 2017 [20–22]. GLA-SE, an emulsion-based TLR4 agonist, evaluated within an RSV postfusion F protein subunit vaccine improved immunogenicity in older adults [23, 24], but failed to protect recipients from RSV disease in a subsequent study [25]. Because of the urgent need to combat COVID-19, multiple SARS-CoV-2 vaccines were rapidly developed across various platforms including mRNA-based, viral vector-based, inactivated, and subunit vaccines. Most inactivated and subunit vaccines contain adjuvants such as alum [26]. Of note, the saponin Matrix-M, AS03, and CpG with alum demonstrated promising immunogenicity data among older adults [27, 28].
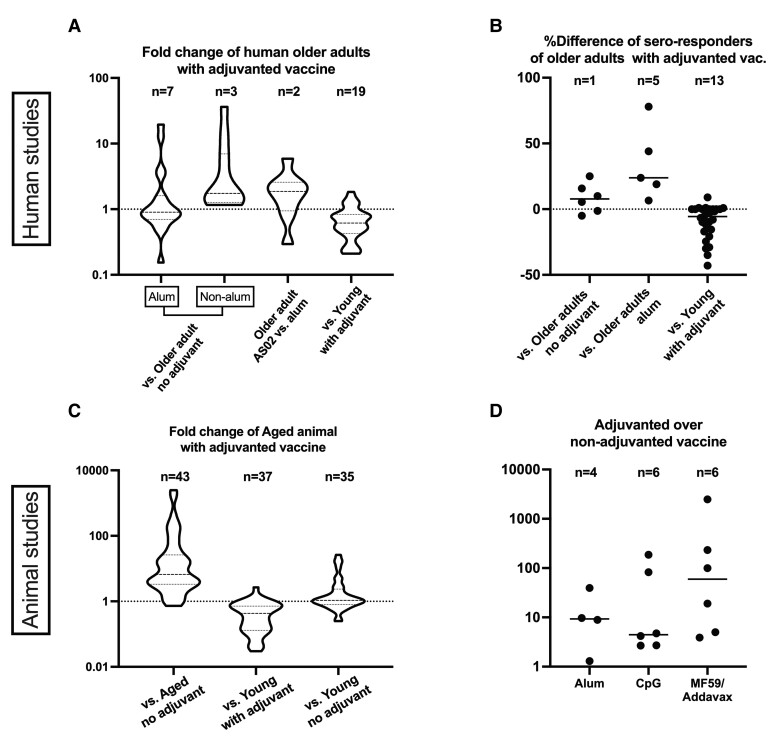
Because of the limited number of studies for each adjuvant–antigen combination in this category, we performed pooled semiquantitative analysis to evaluate the immunogenic effects of adjuvant categories in older adults. The median geometric mean titer ratio (GMTR) of antigen-specific Ab induced by adjuvanted vs nonadjuvanted vaccines was 1.2 (interquartile range [IQR], 0.8–2.4). Adjuvants were further categorized as alum or non-alum. Although alum did not enhance Ab production compared with no-adjuvant (median GMTR, 0.9; IQR, 0.6–1.3), non-alum adjuvants showed robust activation of humoral immunity with median GMTR of 1.8 (IQR, 1.3–7.1) (Figure 3A). The AS02 adjuvant system also increased Ab responses compared with the conventional alum adjuvant (median GMTR, 1.8; IQR, 1.1–2.5) (Figure 3A). Similarly, seroresponse rates were higher in the adjuvanted vs nonadjuvanted vaccines, and higher with the newer adjuvants such as CpG vs the conventional alum adjuvant (Figure 3B). Studies comparing responses across adult age indicated that older adults demonstrated impaired humoral responses compared to younger adults <60 years of age (Figure 3A and B).
Figure 3.
Effect of vaccine adjuvants on humoral immunity in older populations. Vaccine induced humoral responses of older human adults (A and B) and aged animals (C and D) are depicted as compared over comparator groups. Adjuvanted vaccine studies in human older adults were analyzed with respect to (A) GMT ratio and (B) difference in % of seroresponse rates. A and C, Fold-change of mean antibody values are shown in a violin plot. Horizontal lines represent median and IQRs. D, Adjuvants with ≥3 data records were extracted and presented. B and D, Horizontal lines represent medians. Each dot represents a comparison. Some human studies included >1 comparison (eg, antibody titers for different pneumococcal antigens). Seroresponse rates differed by antigen and were defined as follows: ≥3-fold rise over baseline in anti-F IgG for RSV studies, ≥4-fold increase in Ab titers for C difficile studies, anti-HBs >10 mIU/mL for hepatitis B studies, neutralizing antibody titers of ≥1:10 for Ross River Virus studies, neutralizing antibody titers against diphtheria >1 IU/mL for Tdap studies. Abbreviations: C diff, Clostridium difficile; GMT, geometric mean titer; RSV, respiratory syncytial virus; Tdap, tetanus, diphtheria, and pertussis.
Human in Vitro Studies
Only 3 articles assessed the effect of adjuvants in older adult populations using human in vitro modeling. Distinct responses of antigen-presenting cells (APCs) have been noted by age, showing varying degrees of synergy across elders, adults, and infants, when testing multiple combination adjuvants [29]. Remarkably, a TLR/C-type lectin receptor (CLR) activating adjuvant combination tested in vitro was synergistic in activating infant APCs, additive toward middle age APCs, and actually antagonistic toward leukocytes of older adults. The TLR4 agonist GLA efficiently increased expression of costimulatory molecules and production of proinflammatory cytokines on human dendritic cells (DCs) derived from young and older adults with no age-related differences, suggesting that TLR4 agonists are promising candidates as adjuvants tailored to older adults [30]. Furthermore, via in vitro activation of human leukocytes from older adults, unique immunological properties of the alum:CpG adjuvant formulation were observed that demonstrated synergistically enhances proinflammatory cytokines from human peripheral blood mononucleolar cells and monocyte derived DC-dependent memory T-cell activation [31].
Animal Studies
We identified 64 articles that evaluated the effect of adjuvants in aged animal models (Figure 1). Nearly all studies (n = 59, 92%) used aged mice (9 to 24 months old), whereas some used aged pig (n = 2, >3-year-old), nonhuman primate (n = 1, ≥18-year-old), rat (n = 1, 20-month-old), or domestic sheep (n = 1, 3-year-old) models. Half of the studies (n = 33, 52%) focused on vaccines for influenza, followed by pneumococcus (n = 4), RSV (n = 3) and SARS-CoV-2 (n = 3). Adjuvants evaluated are shown in Figure 2. Compared with the adjuvants evaluated in human clinical studies, we noted that a broader variety of adjuvants were tested in animal studies. PRR agonists (alone or formulated with O/W emulsion, alum, and others) were the most extensively studied adjuvant category (n = 39, 39%) with TLR9 ligand CpG-ODN most frequently applied (n = 11, either alone or formulated with other components). More than half (n = 36, 56%) of these preclinical studies only evaluated one adjuvant, and nearly one-quarter evaluated 2 or 3 adjuvants (n = 14, 22%, and n = 13, 20%, respectively) (Figure 2B). Only 1 study compared >3 adjuvants and only 5 studies (8%) tested multiple adjuvant doses. The immunization schedule applied to aged animal studies was either a single fixed schedule (n = 55, 86%), or multiple dose schedule (n = 9, 14%). Humoral immunity was the most frequently evaluated readout of immunogenicity (n = 53, 83%), followed by cell-mediated immunity (n = 31, 48%). Vaccine efficacy was also evaluated with infectious challenge in half (n = 32, 50%) of the studies.
The effects of age and adjuvant type on Ab titer are summarized in Figure 3C and D. Overall, adjuvants improved immunogenicity in aged animals compared with nonadjuvanted vaccines (median fold-change, 6.6; IQR, 3.3–26.1). When administered to aged animals, the same adjuvanted vaccine elicited lower Ab titers compared with young animals (median fold change, 0.43; IQR, 0.13–0.72). Interestingly, Ab levels of aged animals immunized with adjuvanted vaccines was comparable to those of young animals immunized with nonadjuvanted vaccines (median fold change, 1.1; IQR, 0.80–2.4). These data suggest that adjuvants can overcome the dampened humoral response of older animals.
DISCUSSION
This scoping review is the first to systematically assess both preclinical and clinical studies of adjuvants in the aged. This effort demonstrated that a variety of adjuvants have been evaluated in vaccine studies in human older adults and aged animals (Table 1). Importantly, several adjuvant-antigen combinations had shown to be effective in older adults. The O/W emulsions MF59 and AS03 have been used as adjuvants in pandemic and seasonal influenza vaccines for older adults since the late 1990s, with greater effectiveness compared to standard influenza vaccines [16, 32, 33]. Newer adjuvants including TLR4 and TLR9 agonists (eg, AS02, GLA-SE, CpG) have recently been developed and demonstrate enhanced humoral immunity in pneumococcal, RSV, and hepatitis B vaccines for this population [20, 21, 24, 34]. Of note, CpG-adjuvanted hepatitis B vaccine demonstrated superiority over conventional alum-adjuvanted hepatitis B vaccine in older adults even with a dose-sparing regimen [21, 22]. Combination adjuvant systems, developed by GlaxoSmithKline, contain more than 1 class of adjuvant and include AS01 (MPL + QS21 in liposomes), AS02 (MPL + QS21 in O/W emulsion), AS03 (alpha-tocopherol in O/W emulsion), and AS04 (MPL + alum) [35]. AS01-adjuvanted HZ/su vaccine has demonstrated remarkable real-world effectiveness with a favorable safety profile and is currently approved in >30 countries [36]. Encouraging preclinical and clinical data support a growing pipeline of novel adjuvants tailored to older adults [31]. In contrast, our review indicated that the conventional alum adjuvant did not consistently enhance humoral immune responses in older adults when evaluated in pneumococcal, RSV, influenza, and Clostridium difficile vaccines, which highlights the need for new adjuvants that are active in older adults [10, 18, 37–39].
Table 1.
Adjuvants to Enhance Vaccine Responses in Older Adults: Achievements, Current Limitations, and Future Challenges
| Achievements |
|
| Current limitations and unknowns |
|
| Future directions |
|
Vaccines are the most effective public health intervention and commonly target childhood infectious diseases and save millions of lives annually [40]. Recent efforts have focused on expanding vaccination programs to protect additional vulnerable populations such as older adults. Development and deployment of safe and effective vaccines for older adults are undoubtedly crucial; however, vaccines are often less effective in older adults compared with young populations as demonstrated in this review. This is mainly because of the altered immune function associated with aging, also known as immunosenescence, which highlights the need for vaccines tailored to older adults. Most subsets of naïve, memory, and effector T cells, regulatory T cells, and B cells are negatively affected by age [41, 42]. Increasing age also alters innate immune responses. For instance, DCs, which act as critical commanders in the activation and regulation of adaptive immune cells are decreased in number and function in older vs younger adults [42]. One promising approach to restore the impaired immune response of older adults is the use of adjuvants. Mechanism of action varies across adjuvants. For example, alum confers its adjuvanticity in part by (1) depot effect, (2) NLRP3 inflammasome activation, and (3) damage-associated molecular patterns-mediated signaling [6, 43]. The O/W emulsion MF59 induces chemokine production such as CCL2 at the injection site, which amplifies immunostimulatory responses both at the injection site and draining lymph node. CpG activates TLR9, which results in MyD88 pathway activation and type I interferon response [6]. AS01 is a liposome formulation containing MPL and saponin QS21 that synergistically activate the immune system largely through TLR4 and caspase 1 activation [43]. Despite an increasing number of available adjuvants with different mechanisms of action, more studies are needed to determine which adjuvant can most effectively counteract the impaired vaccine immune responses of older adults (Table 1).
Because of distinct immune responses induced by different immunogens, optimal antigen/adjuvant combinations will likely vary by pathogen target. Furthermore, correlates of protection differ between diseases. Our review demonstrated several gaps within this area. Although a growing number of novel adjuvants has been studied, the number of adjuvants compared in any particular study was limited. Most studies only evaluated a single adjuvant and no study compared more than 3 adjuvants in human clinical trials. The use of different antigens, heterogeneous human and animal populations, and nonstandardized outcome measures make it difficult to compare immunogenicity and efficacy across studies. Although designing large-scale human clinical studies directly comparing multiple adjuvants in the same target population can be impractical and costly, preclinical animal models lend themselves to such a systematic approach. Given the distinct immune characteristics of older adults, using aged animals to test multiple adjuvants head to head may help accelerate vaccine development for older adults (Table 1). More research is needed to assess the correspondence of immunosenescence in nonhuman species to that of humans, while acknowledging that no one animal model will suit all study needs.
Four factors are creating a major bottleneck in developing optimal adjuvants for older adults: (1) the growing pipeline of candidate adjuvants, in part supported by the National Institute of Allergy and Infectious Diseases Adjuvant Discovery Program [10]; (2) species-specific differences in immune signaling pathways; (3) in vivo evaluation systems in animals and humans that compare at most 2 or 3 adjuvants at a time; and (4) adjuvant combinations/adjuvantation systems can have distinct combinatorial effects that vary by demographic features such as age [35]. This scenario highlights the potential relevance of in vitro models as a promising high-throughput platform to study multiple innate immune signaling pathways in a species-specific (ie, human) and age-specific (ie, older adults) manner, accelerating and derisking discovery and development of adjuvants for older adults (Table 1). Vaccine development has traditionally moved slowly in part because of to disregarding species and age specificity during preclinical studies. In this context, human in vitro systems that recapitulate innate and adaptive immune responses have demonstrated ability to model age-specific immunity in newborns, infants, and middle-aged adults [44–47]. Multiple lines of evidence suggest that such in vitro systems generate data that are relevant in vivo. For example, the action of licensed (self)-adjuvanted vaccines mirrors that observed in human clinical trials [46], and adjuvants identified as highly active toward primary human infant leukocytes in vitro proved highly effective in neonatal Rhesus macaques in vivo [45]. However, our search strategy yielded only 3 articles modeling adjuvant responses of older adults in vitro [29–31], demonstrating that this area is relatively understudied and merits further exploration.
Increased mortality and morbidity of older adults during the COVID-19 pandemic further highlighted importance of including special populations in the vaccine development and testing process. Licensed SARS-CoV-2 mRNA vaccines represent key milestones as they dramatically accelerated the process of vaccine development and are effective at preventing severe COVID-19 in older adults. Despite their success, challenges related to scaling production and ultra-cold storage have limited worldwide access to these vaccines. In this context, adjuvanted protein subunit vaccines may serve an important complementary role given their stability, established technology, and long-standing safety across ages including older adults and young children. Evaluating multiple adjuvants at the same time in the same target population is critical to accelerate vaccine development during the preclinical phase. A nonhuman primate study compared immune responses and disease protection of five adjuvant formulations for SARS-CoV-2 subunit vaccines and demonstrated the importance of head-to-head adjuvant comparison [48]; however, it was limited to young adult animals. To find the optimal antigen-adjuvant combination for older adults, we evaluated 8 adjuvant formulations for SARS-CoV-2 protein subunit vaccines in aged mice, and found that alum and CpG were highly immunogenic and protective [31]. We also demonstrated that alum and CpG induced robust innate immune responses in peripheral blood mononuclear cells isolated from human older adults. These observations represent the conceptual underpinnings of a vaccine such as Corbevax (trial no. CTRI/2020/11/029032). Similar approaches can be considered for future vaccine studies.
Our scoping review is the first such study to provide a systematic and semiquantitative evaluation of adjuvants for older adults. We note several limitations of this review. First, we were limited in our data synthesis to the assessment of humoral immunity because of the paucity and methodological heterogeneity of studies exploring cell-mediated immunity. Cellular immunity is also important for vaccine efficacy, as evidenced in the adjuvanted subunit zoster vaccine studies, and may be more relevant for protection from a given disease. For example, an enhanced humoral response to a GLA-SE-adjuvanted RSV vaccine did not correlate with efficacy in a phase 2b trial [25, 49]. Second, significant heterogeneity of study protocols and methods limited statistical comparisons between adjuvants to relative fold-changes in Ab production. However, adjuvant enhancement of humoral immunity in the aged was generally consistent across animal and human studies. Third, we did not account for antigen-adjuvant combinations in this review. Because an adjuvant may act differently when paired with different antigens, we cannot generalize the results generated from one antigen-adjuvant combination to others.
In conclusion, known and novel adjuvants have the potential to enhance vaccine-induced immunity in older adults. Standardized, systematic approaches to select the optimal adjuvant type and dose in the early, preclinical stages of vaccine development, including potential use of age-specific human in vitro and animal models, should be considered to help accelerate translation and discovery of safe and effective adjuvanted vaccines tailored for immunologically distinct populations, such as older adults (Table 1).
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Etsuro Nanishi, Precision Vaccines Program, Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA; Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, USA.
Asimenia Angelidou, Precision Vaccines Program, Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA; Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, USA; Department of Neonatology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Chloe Rotman, Medical Library, Boston Children’s Hospital, Boston, Massachusetts, USA.
David J Dowling, Precision Vaccines Program, Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA; Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, USA.
Ofer Levy, Precision Vaccines Program, Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA; Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, USA; Broad Institute of MIT & Harvard, Cambridge, Massachusetts, USA.
Al Ozonoff, Precision Vaccines Program, Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA; Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, USA; Broad Institute of MIT & Harvard, Cambridge, Massachusetts, USA.
Notes
Acknowledgments. The authors thank the members of the BCH Precision Vaccines Program for helpful discussions. We thank Drs. Kevin Churchwell, Gary Fleisher, and David Williams, and Mr. August Cervini for their support of the Precision Vaccines Program. D. J. D. thanks Siobhan McHugh, Geneva Boyer, Lucy Conetta, and the staff of Lucy’s Daycare, the staff the YMCA of Greater Boston, Bridging Independent Living Together (BILT), Inc., and the Boston Public Schools for childcare and educational support during the COVID-19 pandemic. E. N. is a Japan Society for the Promotion of Science (JSPS) Overseas Research Fellow.
Financial support. This work was supported in part by US National Institutes of Health (NIH)/National Institutes of Allergy and Infectious Diseases (NIAID) awards including Molecular Mechanisms of Combination Adjuvants (1U01AI124284-01), Adjuvant Discovery (HHSN272201400052C and 75N93019C00044) and Development (HHSN272201800047C) Program Contracts, and Human Immunology Project Consortium (HIPC) U19AI118608-01A1 to O. L. D. J. D.’s laboratory is supported by NIH grant (1R21AI137932-01A1), Adjuvant Discovery Program contract (75N93019C00044), and Development of Vaccines for the Treatment of Opioid Use Disorder contract (75N93020C00038). The Precision Vaccines Program is supported in part by the BCH Department of Pediatrics and the Chief Scientific Office.
Supplement sponsorship. This supplement is sponsored by the Precision Vaccines Program of Boston Children’s Hospital.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. United Nations, Department of Economic and Social Affairs, Population Division . World Population Ageing 2019. Available at: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf. Accessed 31 March 2022.
- 2. Centers for Disease Control and Prevention (CDC) . COVID-19 Hospitalization and Death by Age. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html. Accessed 31 March 2022.
- 3. Gustafson CE, Kim C, Weyand CM, Goronzy JJ. Influence of immune aging on vaccine responses. J Allergy Clin Immunol 2020; 145:1309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soni D, Van Haren SD, Idoko OT, et al. . Towards precision vaccines: lessons from the second international precision vaccines conference. Front Immunol 2020; 11:590373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19:1597–608. [DOI] [PubMed] [Google Scholar]
- 6. Pulendran B, Arunachalam PS, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov 2021; 20:454–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wagner A, Weinberger B. Vaccines to prevent infectious diseases in the older population: Immunological challenges and future perspectives. Front Immunol 2020; 11:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Pasquale A, Preiss S, Tavares Da Silva F, Garçon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines 2015; 3:320–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reed SG, Tomai M, Gale MJ Jr. New horizons in adjuvants for vaccine development. Curr Opin Immunol 2020; 65:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. 2018 NIAID Strategic Plan for Research on Vaccine Adjuvants. Available at: https://www.niaid.nih.gov/sites/default/files/NIAIDStrategicPlanVaccineAdjuvants2018.pdf. Accessed 31 March 2022.
- 11. Cunningham AL, Lal H, Kovac M, et al. . Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375:1019–32. [DOI] [PubMed] [Google Scholar]
- 12. Lal H, Cunningham AL, Godeaux O, et al. . Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 13. Cunningham AL, Heineman TC, Lal H, et al. . Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J Infect Dis 2018; 217:1750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinberg A, Kroehl ME, Johnson MJ, et al. . Comparative immune responses to licensed herpes zoster vaccines. J Infect Dis 2018; 218:S81–7. [DOI] [PubMed] [Google Scholar]
- 15. Tricco AC, Zarin W, Cardoso R, et al. . Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis. BMJ 2018; 363:k4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ng TWY, Cowling BJ, Gao HZ, Thompson MG. Comparative immunogenicity of enhanced seasonal influenza vaccines in older adults: a systematic review and meta-analysis. J Infect Dis 2019; 219:1525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coleman BL, Sanderson R, Haag MDM, McGovern I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir Viruses 2021; 15:813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang K, Wu X, Shi Y, Gou X, Huang J. Immunogenicity of H5N1 influenza vaccines in elderly adults: a systematic review and meta-analysis. Hum Vaccin Immunother 2021; 17:475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baay M, Bollaerts K, Verstraeten T. A systematic review and meta-analysis on the safety of newly adjuvanted vaccines among older adults. Vaccine 2018; 36:4207–14. [DOI] [PubMed] [Google Scholar]
- 20. Sablan BP, Kim DJ, Barzaga NG, et al. . Demonstration of safety and enhanced seroprotection against hepatitis B with investigational HBsAg-1018 ISS vaccine compared to a licensed hepatitis B vaccine. Vaccine 2012; 30:2689–96. [DOI] [PubMed] [Google Scholar]
- 21. Janssen JM, Jackson S, Heyward WL, Janssen RS. Immunogenicity of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in subpopulations of healthy adults 18-70 years of age. Vaccine 2015; 33:3614–8. [DOI] [PubMed] [Google Scholar]
- 22. Jackson S, Lentino J, Kopp J, et al. . Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine 2018; 36:668–74. [DOI] [PubMed] [Google Scholar]
- 23. Falloon J, Ji F, Curtis C, et al. . A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine 2016; 34:2847–54. [DOI] [PubMed] [Google Scholar]
- 24. Falloon J, Talbot HK, Curtis C, et al. . Dose selection for an adjuvanted respiratory syncytial virus F protein vaccine for older adults based on humoral and cellular immune responses. Clin Vaccine Immunol 2017; 24:e00157-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Falloon J, Yu J, Esser MT, et al. . An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis 2017; 216:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jara A, Undurraga EA, González C, et al. . Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med 2021; 385:875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heath PT, Galiza EP, Baxter DN, et al. . Safety and efficacy of NVX-CoV2373 covid-19 vaccine. N Engl J Med 2021; 385:1172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richmond P, Hatchuel L, Dong M, et al. . Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021; 397:682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Haren SD, Dowling DJ, Foppen W, et al. . Age-specific adjuvant synergy: Dual TLR7/8 and mincle activation of human newborn dendritic cells enables Th1 polarization. J Immunol 2016; 197:4413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weinberger B, Joos C, Reed SG, Coler R, Grubeck-Loebenstein B. The stimulatory effect of the TLR4-mediated adjuvant glucopyranosyl lipid A is well preserved in old age. Biogerontology 2016; 17:177–87. [DOI] [PubMed] [Google Scholar]
- 31. Nanishi E, Borriello F, O’Meara TR, et al. . An aluminum hydroxide:CpG adjuvant enhances protection elicited by a SARS-CoV-2 receptor binding domain vaccine in aged mice. Sci Transl Med 2022; 14:eabj5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weinberger B. Adjuvant strategies to improve vaccination of the elderly population. Curr Opin Pharmacol 2018; 41:34–41. [DOI] [PubMed] [Google Scholar]
- 33. Domnich A, Arata L, Amicizia D, Puig-Barberà J, Gasparini R, Panatto D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: a systematic review and meta-analysis. Vaccine 2017; 35:513–20. [DOI] [PubMed] [Google Scholar]
- 34. Pauksens K, Nilsson AC, Caubet M, et al. . Randomized controlled study of the safety and immunogenicity of pneumococcal vaccine formulations containing PhtD and detoxified pneumolysin with alum or adjuvant system AS02V in elderly adults. Clin Vaccine Immunol 2014; 21:651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin Immunol 2018; 39:14–21. [DOI] [PubMed] [Google Scholar]
- 36. Harbecke R, Cohen JI, Oxman MN. Herpes zoster vaccines. J Infect Dis 2021; 224:S429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Juergens C, de Villiers PJ, Moodley K, et al. . Safety and immunogenicity of 13-valent pneumococcal conjugate vaccine formulations with and without aluminum phosphate and comparison of the formulation of choice with 23-valent pneumococcal polysaccharide vaccine in elderly adults: a randomized open-label trial. Hum Vaccin Immunother 2014; 10:1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Langley JM, Sales V, McGeer A, et al. . A dose-ranging study of a subunit respiratory syncytial virus subtype A vaccine with and without aluminum phosphate adjuvantation in adults > or =65 years of age. Vaccine 2009; 27:5913–9. [DOI] [PubMed] [Google Scholar]
- 39. Greenberg RN, Marbury TC, Foglia G, Warny M. Phase I dose finding studies of an adjuvanted Clostridium difficile toxoid vaccine. Vaccine 2012; 30:2245–9. [DOI] [PubMed] [Google Scholar]
- 40. Black S, De Gregorio E, Rappuoli R. Developing vaccines for an aging population. Sci Transl Med 2015; 7:281ps8. [DOI] [PubMed] [Google Scholar]
- 41. Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol 2018; 40:83–94. [DOI] [PubMed] [Google Scholar]
- 42. Thomas-Crusells J, McElhaney JE, Aguado MT. Report of the ad-hoc consultation on aging and immunization for a future WHO research agenda on life-course immunization. Vaccine 2012; 30:6007–12. [DOI] [PubMed] [Google Scholar]
- 43. O’Hagan DT, Lodaya RN, Lofano G. The continued advance of vaccine adjuvants - ‘we can work it out’. Semin Immunol 2020; 50:101426. [DOI] [PubMed] [Google Scholar]
- 44. Philbin VJ, Dowling DJ, Gallington LC, et al. . Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol 2012; 130:195–204.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dowling DJ, Scott EA, Scheid A, et al. . Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J Allergy Clin Immunol 2017; 140:1339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sanchez-Schmitz G, Stevens CR, Bettencourt IA, et al. . Microphysiologic human tissue constructs reproduce autologous age-specific BCG and HBV primary immunization in vitro. Front Immunol 2018; 9:2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sanchez-Schmitz G, Morrocchi E, Cooney M, et al. . Neonatal monocytes demonstrate impaired homeostatic extravasation into a microphysiological human vascular model. Sci Rep 2020; 10:17836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arunachalam PS, Walls AC, Golden N, et al. . Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature 2021; 594:253–8. [DOI] [PubMed] [Google Scholar]
- 49. Fries L, Shinde V, Stoddard JJ, et al. . Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun Ageing 2017; 14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.