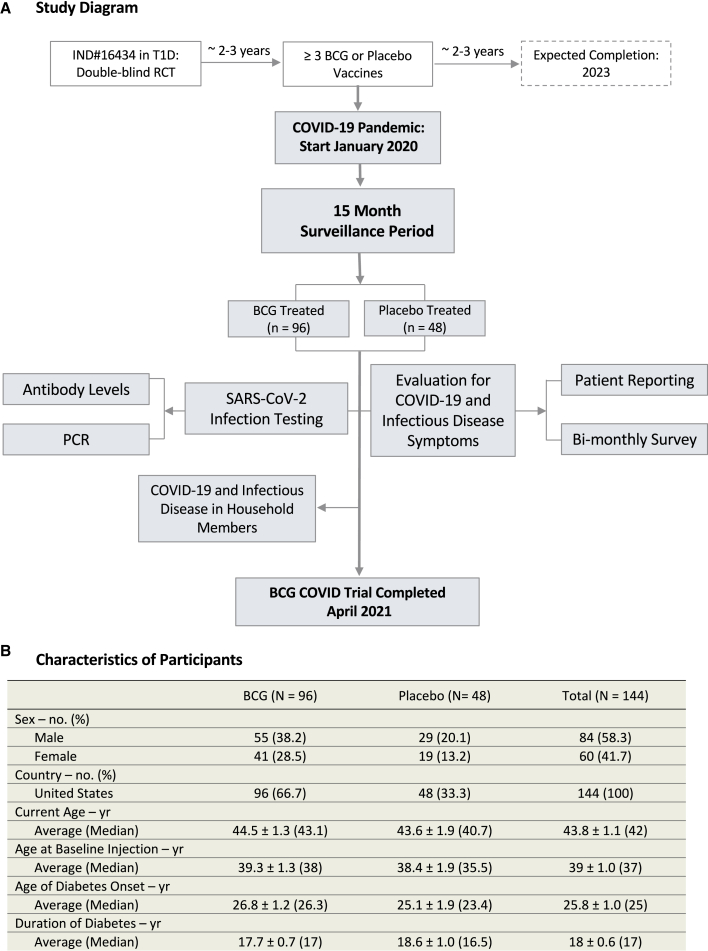
Figure 1.
Flow diagram and characteristics of participants
(A) Flow diagram representing all enrolled participants from January 1, 2020 to April, 2021 for this double-blinded, randomized clinical trial testing repeat Tokyo-172 BCG vaccination versus placebo for COVID-19 protection. All 144 subjects were followed for 15 months with a 2:1 randomization and no dropouts. Data collection for this trial ended on April 2, 2021, the date when subjects started to receive provisionally approved COVID-19-specific vaccines. The geographical locations of the participants within the United States are shown in Figure S5.
(B) Table of participant characteristics.