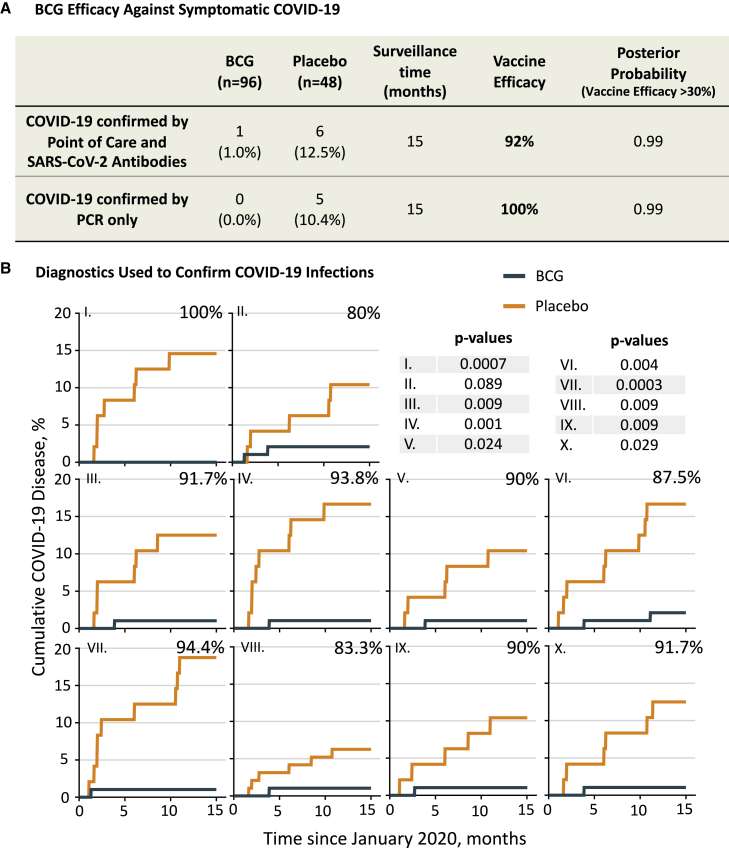
Figure 2.
BCG vaccine efficacy and diagnostic confirmation of COVID-19
(A) Shown is the cumulative incidence of confirmed COVID-19 as a primary endpoint. During the 15-month surveillance time, one BCG recipient out of 96 (1.0%) met our criteria for molecularly confirmed COVID-19. In contrast, 6 out of 48 placebo recipients met the criteria (12.5%). Fisher’s exact testing showed a significant difference (two-tailed p = 0.006). Calculated vaccine efficacy was 92%, and the posterior probability (vaccine efficacy >30%) was 0.99. This was calculated using the Monte Carlo method. Vaccine efficacy was defined as (p1 – p2)/p1 × 100, where p1 is the percentage of COVID-positive subjects in the placebo group and p2 is the percentage of COVID-positive subjects in the BCG group. Our criteria for confirmed COVID-19 (see main text) required a combination of COVID-19 symptom(s) and ≥5 of 10 positive antibody assays including PCR, if available. Since many current clinical trials only define confirmed COVID-19 by symptom(s) and positive PCR testing, the PCR-only group was studied separately for vaccine efficacy (A and B).
(B) Cumulative findings from each of the 10 diagnostic tests used to confirm COVID-19 (along with positive symptoms). These tests included the presence of COVID-19-specific antibodies to various SARS-CoV-2 virus epitopes through protein display (I–VIII), antibodies to the receptor-binding domain with an ELISA test (IX), and point-of-care testing (X) that included PCR. Our criteria for having confirmed COVID-19 required at least 5 of 10 detection methods to be positive, along with symptom(s). For the antibody assays, a patient was considered positive when the test resulted in a Z score of ≥3. In the cumulative graphs, the x axis data show the time period of the 15-month trial. The y axis shows the cumulative percentage of positive subjects. Except for the point-of-care graph (X), all other graphs represent the percentage of BCG and placebo patients with a Z score of ≥3 for the anti-SARS-CoV-2 antibody binding to a given protein region of the virus, i.e., the average antibody level during the COVID trial period was at least 3 standard deviations greater than the average level in the period preceding the COVID trial (baseline). The percentiles at the top right of each graph represent the calculated vaccine efficacy if this test alone was used to diagnose COVID-19 disease. Respective efficacy and Fisher’s exact p values for each COVID-19 antibody test were as follows: (I) 100%, p = 0.0007; (II) 80%, p = 0.089; (III) 91.7%, p = 0.009; (IV) 93.8%, p = 0.001; (V) 90%, p = 0.024; (VI) 87.5%, p = 0.004; (VII) 94.4%, p = 0.0003; (VIII) 83.3%, p = 0.009, (IX) 90%, p = 0.009; and (X) 91.7%, p = 0.029. For all graphs, BCG n = 96, placebo n = 48.
Figure S1 shows the viral protein regions for each anti-COVID antibody tested. Number at risk data for each cumulative graph are shown in Figure S4.