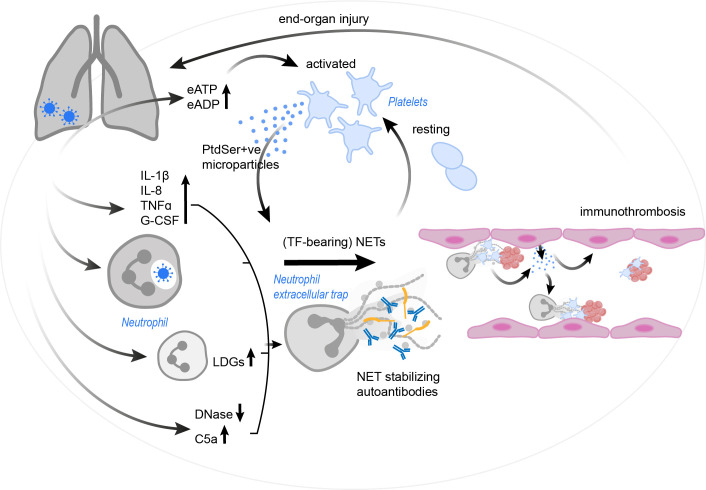
Figure 4.
The vicious cycle of platelet-neutrophil interplay contributing to immunothrombosis in COVID-19. SARS-CoV-2 infection results in a drastic increase in pro-inflammatory cytokines and complement C5a, the occurrence of LDGs and a reduction in endogenous DNases. These effects, as well as the virus itself (via currently unknown mechanisms) may trigger and/ or enhance NET formation. Autoantibodies may further stabilize the formed NETs and protect them against removal. NETs in COVID-19 patients were found to have incorporated procoagulant tissue factor. Platelets are activated by the NETs, but also by an increase in eATP and eADP. Platelets might then react with the production of PtdSer+ve microparticles, which in turn may trigger NETosis and drive coagulation. Endothelial cells also release microparticles, stimulating neutrophils for NET formation. The resulting immunothrombosis very likely contributes to significant end-organ damage. C5a, complement C5a; COVID-19, corona virus disease 2019; DNase, deoxyribonuclease; eADP, extracellular adenosine diphosphate; eATP, extracellular adenosine triphosphate; G-CSF, granulocyte-colony stimulating factor; IL, interleukin; LDG, low density granulocyte; NET, neutrophil extracellular trap; PtdSer+ve, phosphatidylserine positive; TF, tissue factor; TNFα, tumor necrosis factor α; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.