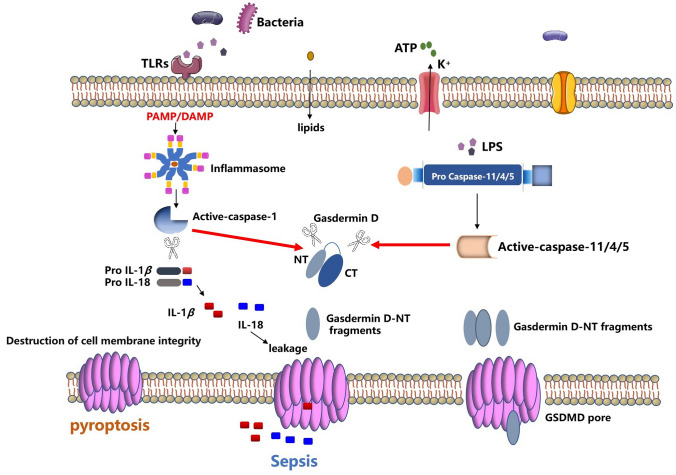
Fig. 1.
An overview of the molecular mechanisms of pyroptosis. Pyroptosis is a type of cell death mediated by caspase-specific proteases (caspase-specific proteases). The canonical pathway of pyroptosis mainly depends on caspase-1. PAMPs and DAMPs associated molecular patterns in the cytoplasm can stimulate inflammasome assembly, leading to the processing and activation of caspase-1, which converts pro-IL-1β and pro-IL-18 cleavage to mature form and cleavage of gasdermin D. The non-canonical pathway of pyroptosis mainly depends on caspase-11 (the human homologous caspase-4/5), activation of caspase 11/4/5 does not normally require PRR-mediated inflammasome, caspase-11 can directly recognize LPS in the cytoplasm for oligomerization and activation, disrupt the membrane structure by cleaving and activating GSDMD. GSDMD has two conserved domains: an N-terminal effector domain and a C-terminal inhibitory domain. The N-terminal is the main functional domain and is involved in the occurrence of pyroptosis, while the C-terminal has an auto-inhibitory function. In the resting state, the N-terminal and C-terminal domains are connected by a long loop. When stimulated by an external signal, Caspase-1/4/5/11 activates the cleavage of GSDMD protein, and GSDMD-NT is activated to bind to the cell membrane. The phospholipid protein binds, multimerizes, and forms a hole in the cytoplasmic membrane, which damages the cytoplasmic membrane and induces the occurrence of pyroptosis. At the same time, activated forms of pro-inflammatory cytokines such as IL-1β and IL-18 are released through the hole, leading to a massive inflammatory response