Abstract
Introduction: Partial caries removal has been shown to be an effective method to treat deep carious lesions in deciduous teeth. Nevertheless, the possibility of keeping infected dentin in the cavity still requires additional investigation. The objective of this research was to describe changes in primary infected dentin after restoration with glass ionomer cement. Methods: Dentin from 45 primary molars with deep and active carious lesions was evaluated using clinical and laboratory criteria, before and 60 days after restoration. The clinical analysis evaluated dentin colour (CO), dentin consistency (COS) and laser fluorescence (LF). The laboratory procedures assessed bacterial contamination and mineral content (MC), and evaluated the dentin ultrastructure and collagen content. Data on CO, COS, LF and colony forming units were analysed using the Wilcoxon signed-rank test; MC, bacterial counts and collagen evaluations were evaluated using the Student’s t-test. Results: After 60 days, lower values of LF were observed, together with a lower bacterial count, and a higher COS was found, with an increase in calcium, phosphorus and collagen contents. Differences were not detected for CO or for fluorine content. Baseline samples showed enlarged tubules with bacterial invasion; 60-day samples showed better organised tissue, with a more compact intertubular dentin and narrower tubules. Conclusion: It is concluded that appropriate cavity sealing can promote beneficial changes in deep carious lesions of primary teeth, even in the presence of infected dentin.
Key words: Tooth, deciduous, dental restoration, deep carious lesions
INTRODUCTION
Most studies on partial caries removal show histological and clinically positive changes after restoration when affected dentin is left in the cavity. Better tissue organisation and a significant reduction in bacterial counts1., 2., 3., 4. are seen, leading to thicker intertubular dentin and a more compact collagen network5, without caries progression. Clinical research with longitudinal follow-up confirms these findings, as radiographic evidence is compatible with tertiary dentin formation6., 7..
However, it must be pointed out that the selective removal of decayed tissue, as preconised by the papers cited above, poses some difficulty for clinicians8., 9.. There is no reliable way to differentiate the two layers of carious dentin in daily practice9 because the criteria used are based on an operator’s visual (colour) and tactile (hardness) perceptions, and thus are highly subjective10.
One may question if all infected dentin is being removed during cavity preparation or, on the other hand, if practitioners are over-preparing cavities. In primary teeth, it is fundamental to retain as much dental tissue as possible in order to avoid pulp exposure and preserve pulp vitality until exfoliation. Thus, an intriguing question is: how important is the total removal of infected dentin to achieve a successful restoration?
Phonghanyudh et al.11 reported successful results after 12 months in primary teeth when partial caries removal was restricted to the enamel-dentine junction. Successful outcomes were also achieved after 5 years in primary teeth treated with stainless steel crowns, without infected dentin removal12. Glass ionomer cements (GICs) have been studied as the restorative material of choice for the remineralisation of caries-affected dentin13. However, to the best of our knowledge, there is still a lack of information in the literature about the changes that take place in sealed infected dentin. Therefore, the aim of this study was to characterise the changes in primary dentin after cavity sealing with a GIC without removal of the infected dentin.
METHODS
The present study received approval from the Ethics Committee of the State University of Ponta Grossa (Ponta Grossa, Paraná, Brazil) under protocol #15821/09.
Sample selection
After an initial screening of approximately 850 schoolchildren, 33 patients of both genders, with ages ranging from 3 to 10 years (average = 6.0 ± 2.1 years), were selected to obtain a power of 80% at a level of significance of 5%. The inclusion criteria were children having at least one second primary molar with an open and active carious lesion in dentin (ICDAS code 6 – Obvious loss of tooth structure, the cavity is both deep and wide and dentin is clearly visible on the walls and at the base. An extensive cavity involves at least half of a tooth surface14), without signs or symptoms of pulpal pathology, checked by clinical and radiographic examination. All lesions were deep (two-thirds or more of the dentin thickness) and were limited to the occlusal surface. Dentin laser fluorescence (LF) with a value between 89 and 99, measured using the DIAGNOdent device (KaVo, Biberach, Germany), was also an inclusion criterion because high fluorescence values indicate a more infected tissue in a deeper cavity15. Children with systemic disease were excluded. All parents/caregivers were informed about the nature of the study and signed a consent form, according to the Helsinki Declaration (version 2002) and the Resolution #196 (National Health Council, Brazil). The final sample consisted of 45 teeth that met the inclusion criteria.
Study design
The carious dentin of each selected tooth was evaluated twice (before and after cavity sealing – at baseline and at 60 days, respectively), using clinical and laboratory criteria. The clinical criteria were: dentin colour and consistency, and LF readings. The laboratory analysis included microbiological and collagen evaluations, and ultrastructural and mineral analyses.
Clinical and experimental procedures
Each patient attended two clinical sessions. At every session, after local anaesthesia, teeth were: (i) isolated with a rubber dam; (ii) cleaned using a new toothbrush and water; (iii) washed thoroughly with air/water spray; and (iv) air dried without desiccation.
At this point, the clinical characteristics of dentin were evaluated as described later in this section. Then, all carious tissue was removed from the cavosurface margins of the cavity until the enamel dentin junction, in order to promote appropriate cavity sealing. No effort was made to remove the most superficial dentin layer from the pulp floor. The dentin left behind was highly infected and soft on probing. The cavities measured about 7 mm in the mesial-distal direction and 5 mm in the buccal-lingual direction, and were 3 mm in depth.
Samples of infected dentin removed before placement of the restoration (baseline samples) were compared with samples taken 60 days after cavity sealing (experimental samples). To standardise sample collection, the caries lesions were divided into two equivalent portions using a ‘Hollenback’ carver in a buccal-lingual direction1. Fragments of infected dentin from the mesial portion were collected before placement of the temporary restorations. The infected dentin in the distal portion was left in the cavity to be removed 60 days after cavity sealing. Dentin samples were removed using sterile dentin excavators and processed immediately for laboratory analysis. This routine was used for all the criteria investigated.
Teeth were sealed with a high-viscosity GIC (Ketac Molar Easy Mix; 3M ESPE, St Paul, MN, USA). The cement was covered with Adper Single Bond 2 (3M ESPE) to prevent changes in glass ionomer structure immediately after restoration placement. To facilitate reopening of the cavity after 60 days, dentin conditioner was not used at baseline. A single operator (A.C.R.C.) performed all the clinical procedures for standardisation purposes.
The integrity of each restoration was evaluated after 60 days according to the criteria of the United States Public Health Service (USPHS)16: (i) cavosurface marginal discoloration; (ii) recurrent caries; (iii) contour or loss of substance (wear); (iv) marginal integrity; and (v) surface texture. Those variables were ranked according to the following scores: alfa (no defect clinically detectable, needing just a polish); bravo (clinically acceptable, but repair is necessary); and charlie (clinically unacceptable, needs restoration substitution).
To reopen the cavity, the restorative material was carefully removed using sterile diamond burs (#1016; KG Sorensen, São Paulo, SP, Brazil) under water cooling using sterile physiological saline and dentin excavators. At this phase, air spray was constantly applied to make the GIC opaque, which helped to distinguish the restorative material from the dentin tissue. When the GIC layer was very thin, it was removed using dentin excavators only in order to avoid the unintentional removal of the remaining dentin. This protocol has been used before by other researchers1., 5..
The clinical characteristics of the dentin were re-evaluated and fragments of infected dentin from the distal portion (60-day samples) were removed for laboratory analysis. After this, the teeth were restored using Ketac Molar Easy Mix (3M ESPE), according to the manufacturer’s instructions.
Clinical evaluation: dentin colour and dentin hardness on probing
Dentin colour and dentin hardness on probing were evaluated at baseline and 60 days after sealing. Hardness of carious dentin on probing was classified using the following scores: 0, soft (probe penetrates tissue with no resistance when removing probe); 1, medium (slight resistance when removing probe); or 2, hard (comparable to unaffected dentin)4. The colour was evaluated using the following scores: 0, light yellow; 1, yellow; 2, light brown; and 3, brown. Both analyses were performed by a single trained examiner (A.C.R.C.) who achieved a Kappa index of 0.80 for the dentin colour and 0.90 for the dentin-consistency evaluation. Intra-examiner agreement for colour was obtained using clinical photographs; the consistency index was obtained after in vivo examination of carious lesions. The selected cavities used for calibration presented the same characteristics as those in this study.
LF readings
Dentin LF was measured using DIAGNOdent 2095 (KaVo), according to the manufacturer’s instructions. The device was calibrated against a porcelain reference object provided before the examination and then it was recalibrated on a sound surface of every tooth. The possible values of LF range from 0 to 99. Three LF measurements were made of dentin in the mesial (baseline measurement) or distal (60 days after sealing) portions of the cavity, and these measurements were used to calculate a mean value for each time point (i.e. baseline and 60 days), for each tooth.
Microbiological and collagen evaluation
Dentin fragments from the mesial (baseline sample) and distal (60-day sample) portions were collected and processed immediately for histological analysis. The protocol consisted of fixation in 10% formaldehyde for 48 hours followed by demineralisation in 4.13% ethylenediaminetetraacetic acid (EDTA) for 2 months. After an overnight wash in phosphate-buffered saline (PBS, pH 7.4), each sample was dehydrated in graded ethanol and embedded in paraffin wax. From each dentin sample, sections (of 5 μm thickness) were obtained using a microtome (Leica RM2125; Leica Biosystems, Wetzlar, Germany). These sections were mounted on slides, dewaxed in xylene, rehydrated in graded ethanol and washed thoroughly with distilled water; then, they were stained with haematoxylin and eosin to identify microorganisms and with Masson’s trichrome staining protocol to detect collagen fibres. One trained and blinded evaluator used Image Pro-Plus software (Media Cybernetics, Warrendale, PA, USA) to analyse the slides, counting the pixels of purple (haematoxylin and eosin) and blue (Masson’s trichrome) in each slide. This process was repeated three times, and a mean value was obtained for each slide.
Ultrastructural and mineral analysis
Dentin fragments from the mesial (baseline sample) and distal (60-day sample) portions were stored for 7 days in 0.1 M sodium phosphate buffer containing 2% glutaraldehyde (pH 7.4), rinsed thoroughly with 50% sodium phosphate buffer (0.3 M) and distilled water solution (three 30 minutes rinses) and then dehydrated in acetone. Specimens were mounted on stubs and sputter-coated with a 10-nm gold layer before analysis in a scanning electron microscopy (SEM; Shimadzu XRD 6000; Shimadzu Corporation, Tokyo, Japan) using secondary electron mode. The same sample was analysed using energy dispersive X-ray spectroscopy (EDS). The weight percentages of calcium, phosphorus and fluorine were obtained, before and after cavity sealing, at a magnification of ×200 for 100 seconds.
Statistical analysis
The tooth was used as the experimental unit for data analysis. For dentin colour, dentin consistency and LF, the Shapiro–Wilk and Kolmogorov–Smirnov tests showed non-normal distribution of data and therefore the non-parametric Wilcoxon signed-rank test was used to compare the differences before and after cavity sealing. The paired t-test was used to compare the data from the microbiological and collagen analyses and for the weight percentage of calcium, phosphorus and fluorine. The Statistical Package for Social Sciences (SPSS® 17.0, Chicago, IL, USA) program was used for statistical analyses, with the significance level set at 0.05.
RESULTS
Two teeth were lost: one had pulp exposure on the second clinical session; and the other was not available because the patient had moved away from the area. Therefore the final sample consisted of 43 teeth from 31 patients.
Clinical evaluation
No tooth sensitivity was reported during the study period. The radiographic and clinical analyses revealed no signs of necrosis or pulpitis during the follow-up period. After 60 days, all the provisional restorations were ranked as alfa using the USPHS criteria, confirming that appropriate sealing was obtained throughout the experimental period.
The results of dentin colour, dentin consistency and LF readings are provided in Table 1. No significant difference in dentin colour was observed between the two periods of evaluation (P = 0.366). A significant change in dentin consistency was observed 60 days after cavity sealing (P < 0.0001). An increased resistance to manual removal of dentin tissue could be observed. Statistical differences in LF readings, before and after cavity sealing, were also observed (P < 0.0001).
Table 1.
Dentin colour, dentin consistency and laser fluorescence readings (n = 43)
| Variable | Baseline | 60 days | P value* |
|---|---|---|---|
| Dentin colour | 2 (1.5–2) | 2 (2–3) | 0.366 |
| Dentin consistency | 0 (0–1) | 1 (1–2) | <0.0001 |
| Laser fluorescence | 99 (99–99) | 71 (57–78) | <0.0001 |
Values are given as median (interquartile range).
Wilcoxon signed-rank test.
Microbiological, collagen, ultrastructural and mineral analyses
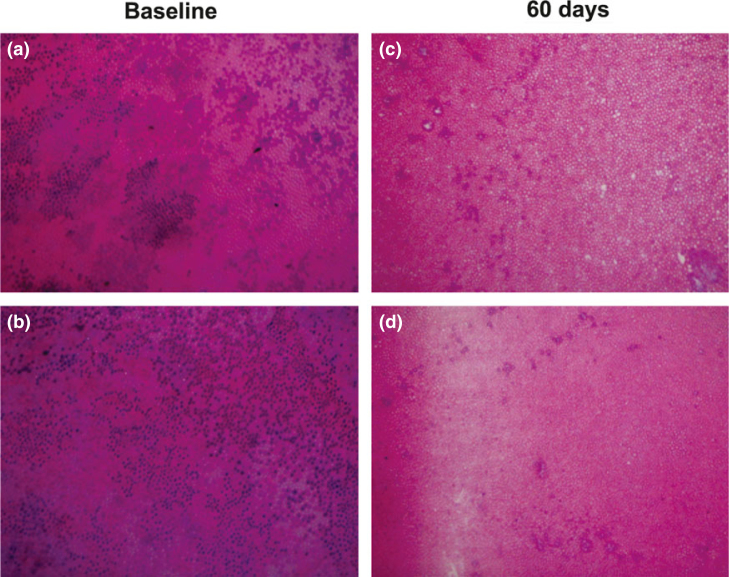
In the qualitative analysis, baseline samples exhibited more infected tissue compared with the 60-day samples, as seen both in SEM microphotography (Figure 1) and in haematoxylin and eosin-stained slides (Figure 2); in the quantitative analysis, this difference was not significant (Table 2).
Figure 1.
Representative secondary scanning electron microscopy (SEM) images of primary carious dentin at baseline (a) and at the 60-day follow-up (c). In baseline samples (a), there is a high degree of dentin demineralisation. Higher magnification of the baseline sample (b) shows exposed collagen fibres (pointer) and highly infected dentin (arrow). After cavity sealing (c), 60-day samples exhibit narrowing of dentinal tubules. The higher magnification (d) of the same image shows evident signs of mineralisation and better organisation of tissue. The bar represents 5 μm (magnification: ×5000).
Figure 2.
Representative images of haematoxylin and eosin-stained slides of deciduous carious dentin. At baseline, microbiological contamination is evident and the presence of microorganisms in the dentinal tubuli is observed (a, b). In panels a and b, there is a strong predominance of purple shades, indicating staining of cell nuclei with haematoxylin as a result of the acidic group in the nucleic acids. After 60 days of sealing, the prevalence of microorganisms is reduced (c, d) and there is predominance of a pink shade because the dentin matrix is composed mainly of proteins which are stained with eosin (magnification: 20×).
Table 2.
Analyses of microorganisms, collagen and mineral in primary caries dentin at baseline and in 60-day samples (n = 43)*
| Variable | Baseline | 60-day | P value |
|---|---|---|---|
| Microbiological analysis (no. of purple pixels)† | 20056.4 (9292.9) | 16060.7 (11944.8) | 0.311 |
| Collagen analysis (no. of blue pixels)‡ | 597401.1 (352709.3) | 1149481.9 (469301.4) | <0.001 |
| Mineral analysis (% weight) | |||
| Calcium | 11.4 (5.3) | 14.5 (4.2) | 0.001 |
| Phosphorus | 9.0 (3.0) | 10.6 (2.3) | 0.002 |
| Fluorine | 1.3 (0.7) | 1.6 (0.8) | 0.067 |
Values are given as mean (standard deviation).
Student’s t-test.
Haematoxylin and eosin stain.
Masson’s trichrome stain.
The analysis of collagen showed a significant increase in the blue pixels of Masson’s trichrome-stained slides, indicating enhanced collagen content after 60 days of sealing (Table 2; Figure 3).
Figure 3.
Representative images of Masson’s trichrome-stained slides of deciduous carious dentin at baseline (a–c) and 60 days after cavity restoration (d–f). The collagen fibrils, stained in shades of blue, showed a significant increase in prevalence after sealing. The more homogeneous shade of blue, seen in panels d, e and f, indicates the presence of intact collagen fibrils after 60 days of sealing.
Mineral gain was detected in the 60-day samples when compared with the baseline samples. Higher percentages of calcium and phosphorus were observed (P < 0.003) in the 60-day samples, and no significant difference was observed in the percentage of fluorine (Table 2).
Ultrastructural analysis using scanning electron microscopy corroborated these findings. Baseline samples showed: (i) enlarged diameter of dentin tubules; (ii) highly infected dentin, with aggregates of bacteria in both intertubular dentin and dentinal tubule openings; and (iii) clearly exposed collagen matrix, arranged in a flaccid network (Figure 1a,b). On the other hand, the 60-day samples showed: (i) substantial reduction in bacteria; (ii) reorganisation of dentin tissue, with a more compact arrangement of collagen fibres; and (iii) narrowing of dentinal tubules (Figure 1c,d).
DISCUSSION
The main difference between the present study and earlier studies that evaluated dentin features after partial caries removal and cavity sealing is that in the present study no attempt was made to remove the infected dentin (except from the cavosurface margin in order to provide appropriate sealing). Restoring deep caries lesions in primary teeth enabled dentin reorganisation when infected dentin remained in the cavity. After 60 days of sealing, carious dentin showed better tissue organisation, substantial reduction in the number of bacteria and narrowing of dentinal tubules compared with baseline. Other researchers reported similar results1., 5. when all infected dentin had been removed.
The design of the present study allowed an effective comparison to be made between baseline and 60-day conditions because both samples were obtained from the same tooth and the same patient. Therefore, individual variations were minimised. Cavity sealing after the follow-up period was evaluated and was considered appropriate, and no signs of pain or pulp alterations were noted clinically or radiographically, except for one tooth that was excluded from the analysis.
Drastic reduction of bacterial counts is a common finding reported in the literature when a caries cavity is sealed1., 2., 3., 17.. This finding was also observed visually in the present investigation. Although the results presented in Figures 1 and 2 did not achieve statistical significance, it is possible to detect a decrease in the presence of microorganisms in the dentin. This is probably because of cavity sealing, which modified the environment and blocked communication with the biofilm12., 18.. The main difference between the results cited previously and this research is the use, in the present study, of histological techniques to quantify microorganisms. We evaluated the presence of bacteria in the carious dentin tissue, rather than their viability. Therefore, even the non-viable bacteria that remained in the dentin tissue after cavity sealing were stained because the bacterial nucleus (DNA) has affinity to haematoxylin and hence the purple pixels from the DNA were quantified by the software.
The 60-day samples showed reduced fluorescence, but the DF values measured were much higher than the values reported for sound teeth or the cut-off limits suggested by the literature as indicative for dentin caries (>38)19. The Diagnodent device is a quantitative method used for caries diagnosis that detects the amount of metabolic by-products produced by bacteria8, mainly protoporphyrin IX, meso-porphyrin and copro-porphyrin. The relatively high second LF reading is probably related to the fact that: (i) porphyrin molecules produced by bacteria are still present when the bacteria die; and (ii) porphyrin decay takes longer than 60 days. This LF device performs well for detecting dentinal caries in primary teeth, showing a positive correlation with lesion depth15. So, the subtle differences in the fluorescence readings between the baseline and the 60-day samples can be interpreted as a reduction in bacterial contamination associated with deep caries lesions, but cannot be considered as a threshold for such a diagnosis.
Dentin colour did not change significantly in this study after cavity sealing. There was a trend for carious dentin to become darker, but this was not significant. Changes in dentin colour from light brown to a darker brown are reported in the literature after cavity sealing2., 3., 17.; however, it seems that these changes take longer to occur than the 60-day period of this study as these reports were observed in studies with follow-up periods longer than 3 months2., 3..
Changes in the mineral content and dentin consistency of samples were also noted. Significantly higher weight percentages of calcium and phosphorus were detected after cavity sealing. This mineral gain is in agreement with the harder dentin consistency observed in the present and in other studies by tactile means2., 3., microhardness measurements20 and EDS analysis5. The changes seen on scanning electron microscopy images after 60 days also corroborate this finding. The exposed collagen fibres and gap zones seen at baseline samples are not visible after 60 days, which is evidence of apatite mineralisation. Other signs of dentin mineralisation can be seen in Figure 1c,d. After 60 days, dentin tubules became narrower with the presence of a mineralised mass composed of apatite aggregates at their entrance.
The dentin–pulp complex is an important factor in dentin tissue repair. The response of odontoblasts to the inflammatory reaction induced by the carious lesion is the production of tertiary dentin21, followed by remodelling of the dentin matrix. This process is mediated by metalloproteinases (MMPs) and non-collagen proteins, such as bone sialoprotein22, which act like a link between collagen fibrils and hydroxyapatite23. Enhanced contents of type I collagen and bone sialoprotein, and increased expression of MMP-8, have already been shown in primary carious dentin after cavity restoration with GIC24.
Therefore, the presence of a collagen network is essential for dentin remineralisation. The Masson’s trichrome stain is specific for collagen fibrils. The intense blue colour on the 60-day slides indicates an enhanced content of collagen, which may reflect odontoblast activity and the capacity for dentin reorganisation. This intense blue colour would not be observed if only the cleaved collagen peptide fragments 1/4 or 3/4 were present in the carious dentin tissue. Similar results were obtained in affected and infected carious dentin after cavity sealing25, regardless of the restorative material used26.
Another interesting finding of this research involved fluorine gain. The main source of fluorine was the GIC used for cavity sealing. The literature reports that GIC can release bioactive molecules to contribute to the process of dentin regeneration27. There are studies which show that fluorine exchange between teeth and GIC occurs in a pattern compatible with dentin remineralisation9. Although an increase in the weight percentage of fluorine was detected after cavity sealing, this increase was not statistically significant, in accordance with the findings of an earlier study5. Whether or not fluorine ion is important for the remineralisation process of carious dentin is yet to be addressed.
The outcomes of the present study were positive for all criteria evaluated. Nevertheless, there are important aspects that must be mentioned, namely the correct diagnosis of the pulp condition and the adequacy of the cavity seal. These factors will lead to clinical and radiographic success over time, as shown in another paper from our research group6.
Although predictions on the long-term outcome are limited, all the findings together indicate that the complete removal of infected dentin before restoration is not an essential step to promote beneficial changes in the lesion environment, and that the protocol suggested may be an option for the treatment of primary teeth with deep caries lesions.
Acknowledgements
This work was supported by the Brazilian research agency Fundação Araucária - Apoio ao Desenvolvimento Científico e Tecnológico do Paraná (grant # 525/10). The funders had no role in the study design, data collection and analysis, the decision to publish or the preparation of the manuscript.
References
- 1.Wambier DS, dos Santos FA, Guedes-Pinto AC, et al. Ultrastructural and microbiological analysis of the dentin layers affected by caries lesions in primary molars treated by minimal intervention. Pediatr Dent. 2007;29:228–234. [PubMed] [Google Scholar]
- 2.Duque C, Negrini Tde C, Sacono NT, et al. Clinical and microbiological performance of resin-modified glass-ionomer liners after incomplete dentine caries removal. Clin Oral Investig. 2009;13:465–471. doi: 10.1007/s00784-009-0304-2. [DOI] [PubMed] [Google Scholar]
- 3.Lula EC, Almeida LJ, Jr, Alves CM, et al. Partial caries removal in primary teeth: association of clinical parameters with microbiological status. Caries Res. 2011;45:275–280. doi: 10.1159/000325854. [DOI] [PubMed] [Google Scholar]
- 4.Bjorndal L, Larsen T, Thylstrup A. A clinical and microbiological study of deep carious lesions during stepwise exc-avation using long treatment intervals. Caries Res. 1997;31:411–417. doi: 10.1159/000262431. [DOI] [PubMed] [Google Scholar]
- 5.Massara ML, Alves JB, Brandão PR. Atraumatic restorative treatment: clinical, ultrastructural and chemical analysis. Caries Res. 2002;36:430–436. doi: 10.1159/000066534. [DOI] [PubMed] [Google Scholar]
- 6.Chibinski ACR, Reis A, Kreich EM, et al. Evaluation of primary carious dentin after cavity sealing in deep lesions: a 10-to 13-month follow-up. Pediatr Dent. 2013;35:107E–112E. [PubMed] [Google Scholar]
- 7.Alves LS, Fontanella V, Damo AC, et al. Qualitative and quantitative radiographic assessment of sealed carious dentin: a 10-year prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:135–141. doi: 10.1016/j.tripleo.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Gurbuz T, Yilmaz Y, Sengul F. Performance of laser fluorescence for residual caries detection in primary teeth. Eur J Dent. 2008;2:176–184. [PMC free article] [PubMed] [Google Scholar]
- 9.Ngo HC, Mount G, Mc Intyre J, et al. Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: an in vivo study. J Dent. 2006;34:608–613. doi: 10.1016/j.jdent.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Iwami Y, Yamamoto H, Hayashi M, et al. Relationship between laser fluorescence and bacterial invasion in arrested dentinal carious lesions. Lasers Med Sci. 2011;26:439–444. doi: 10.1007/s10103-010-0798-5. [DOI] [PubMed] [Google Scholar]
- 11.Phonghanyudh A, Phantumvanit P, Songpaisan Y, et al. Clinical evaluation of three caries removal approaches in primary teeth: a randomised controlled trial. Community Dent Health. 2012;29:173. [PubMed] [Google Scholar]
- 12.Santamaria RM, Innes NP, Machiulskiene V, et al. Caries management strategies for primary molars: 1-yr randomized control trial results. J Dent Res. 2014;93:1062–1069. doi: 10.1177/0022034514550717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aykut-Yetkiner A, Simşek D, Eronat C, et al. Comparison of the remineralisation effect of a glass ionomer cement versus a resin composite on dentin of primary teeth. Eur J Paediatr Dent. 2014;15:119–121. [PubMed] [Google Scholar]
- 14.Ismail AI, Sohn W, Tellez M, et al. The International Caries Detection and Assessment System (ICDAS): an integrated system for measuring dental caries. Community Dent Oral Epidemiol. 2007;35:170–178. doi: 10.1111/j.1600-0528.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 15.Braga MM, Mendes FM, Ekstrand KR. Detection activity assessment and diagnosis of dental caries lesions. Dent Clin North Am. 2010;54:479–493. doi: 10.1016/j.cden.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Barnes DM, Blank LW, Gingell JC, et al. A clinical evaluation of a resin-modified. Glass ionomer restorative material. J Am Dent Assoc. 1995;126:1245–1253. doi: 10.14219/jada.archive.1995.0359. [DOI] [PubMed] [Google Scholar]
- 17.Corralo D, Maltz M. Clinical and ultrastructural effects of different liners/restorative materials on deep carious dentin: a randomized clinical trial. Caries Res. 2013;47:243–250. doi: 10.1159/000345648. [DOI] [PubMed] [Google Scholar]
- 18.Schwendicke F, Dörfer C, Paris S. Incomplete caries removal a systematic review and meta-analysis. J Dent Res. 2013;92:306–314. doi: 10.1177/0022034513477425. [DOI] [PubMed] [Google Scholar]
- 19.Diniz MB, Rodrigues JA, de Paula AB, et al. In vivo evaluation of laser fluorescence performance using different cut-off limits for occlusal caries detection. Lasers Med Sci. 2009;24:295–300. doi: 10.1007/s10103-008-0547-1. [DOI] [PubMed] [Google Scholar]
- 20.Franzon R, Gomes M, Pitoni CM, et al. Dentin rehardening after indirect pulp treatment in primary teeth. J Dent Child (Chic) 2009;76:223–228. [PubMed] [Google Scholar]
- 21.Tjäderhane L, Carrilho MR, Breschi L, et al. Dentin basic structure and composition—an overview. Endod Topics. 2012;20:3–29. [Google Scholar]
- 22.Boushell L, Nagaoka H, Yamauchi M. Increased matrix metalloproteinase-2 and bone sialoprotein response to human coronal caries. Caries Res. 2011;45:453–459. doi: 10.1159/000330601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charadram N, Farahani RM, Harty D, et al. Regulation of reactionary dentin formation by odontoblasts in response to polymicrobial invasion of dentin matrix. Bone. 2012;50:265–275. doi: 10.1016/j.bone.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chibinski A, Gomes J, Camargo K, et al. Bone sialoprotein, matrix metalloproteinases and type I collagen expression after sealing infected caries dentin in primary teeth. Caries Res. 2014;48:312–319. doi: 10.1159/000355302. [DOI] [PubMed] [Google Scholar]
- 25.Pinheiro SL, Frasson AD, Bincelli IN, et al. Study of a morphometric model for histological evaluation of the collagen in dentin carious lesions. J Clin Pediatr Dent. 2008;33:123–126. doi: 10.17796/jcpd.33.2.d27n643xg5532704. [DOI] [PubMed] [Google Scholar]
- 26.Pinheiro SL, Gallassi PC, Saldanha TC, et al. Repairing collagen in dentin carious lesions. Influence of sealing the material: a morphometric study. J Clin Pediatr Dent. 2010;34:223–228. doi: 10.17796/jcpd.34.3.ut5444720r61375h. [DOI] [PubMed] [Google Scholar]
- 27.Ferracane JL, Cooper PR, Smith AJ. Can interaction of materials with the dentin-pulp complex contribute to dentin regeneration? Odontology. 2010;98:2–14. doi: 10.1007/s10266-009-0116-5. [DOI] [PubMed] [Google Scholar]