Figure 8.
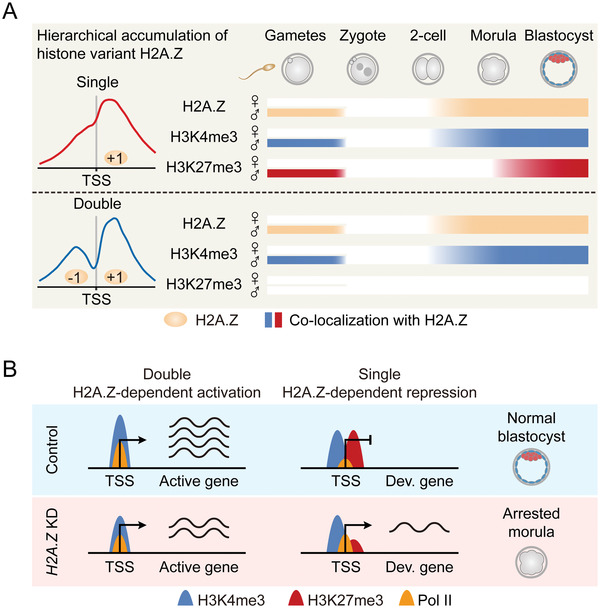
Models of the hierarchical accumulation and function of H2A.Z in early embryos. A) A schematic model showing the reprogramming of H2A.Z from gametes to early embryos. H2A.Z accumulates as “Single” and “Double” peak types at gene promoters in sperm, but exhibits no enrichment in MII oocytes. After fertilization, H2A.Z is globally removed from paternal genome, followed by an unbiased and hierarchical accumulation on parental genomes when H2A.Z is highly transcribed during major ZGA (2‐cell stage). Meanwhile, H3K4me3 accumulates with both “Single” and “Double” H2A.Z peaks in sperm and embryos after ZGA, whereas H3K27me3 prefers to accumulate with “Single” H2A.Z peaks in sperm and embryos at the beginning of lineage commitment (morula stage). B) A schematic model showing the different functions for hierarchical H2A.Z accumulation in early embryos. In normal embryos, “Double” H2A.Z peaks only co‐localize with H3K4me3 at promoters, which facilitate Pol II binding and gene activation. Meanwhile, “Single” H2A.Z peaks colocalize with bivalent marks at promoters, which inhibit Pol II binding and poise expression of development genes (Dev. gene). In H2A.Z KD embryos, decreased H2A.Z accumulation with “Double” peaks is associated with decreased H3K4me3 and Pol II enrichment at promoters, which downregulates active gene expression. Meanwhile, decreased H2A.Z accumulation with “Single” peaks is associated with decreased H3K27me3 enrichment at promoters, which upregulates development gene expression. This dysregulated transcriptional state finally leads to embryonic arrest at the morula stage.
