Figure 7.
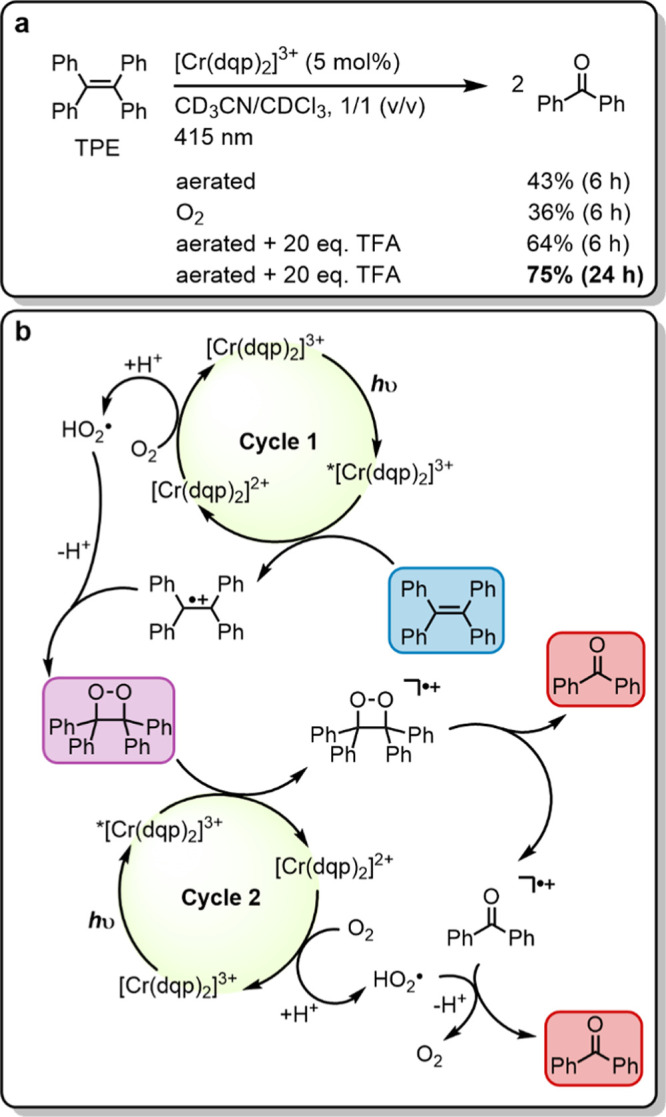
(a) Photocatalytic oxygenation of 1,1,2,2-tetraphenylethene (TPE) with oxygen followed by C–C bond cleavage to two benzophenone units. Irradiation with a 415 nm LED (7.0 W output) with 400 nm long pass filter, 15 mM substrate, 2.0 equiv of 1,4-dichlorobenzene as internal 1H NMR standard, 20 equiv of trifluoroacetic acid (TFA), and 5 mol % of catalyst in 0.6 mL of CD3CN/CDCl3 (1/1, v/v) in closed NMR tubes under air at up to 35 °C (temperature fluctuations caused by the LED radiation). Yields are referenced to the internal standard. (b) Plausible mechanism based on a previous report with 9-mesityl-10-methylacridinium ion (Acr+-Mes) as a photocatalyst.72
