Abstract
Background
Respiratory syncytial virus (RSV) is associated with substantial morbidity in the United States, especially among infants. Nirsevimab, an investigational long-acting monoclonal antibody, was evaluated as an immunoprophylactic strategy for infants in their first RSV season and for its potential impact on RSV-associated, medically attended lower respiratory tract illness (RSV-MALRTI) and associated costs.
Methods
A static decision-analytic model of the US birth cohort during its first RSV season was developed to estimate nirsevimab’s impact on RSV-related health events and costs; model inputs included US-specific costs and epidemiological data. Modelled RSV-related outcomes included primary care and emergency room visits, hospitalizations including intensive care unit admission and mechanical ventilations, and RSV-related mortality.
Results
Under current standard of care, RSV caused 529 915 RSV-MALRTIs and 47 281 hospitalizations annually, representing $1.2 billion (2021 US dollars [USD]) in costs. Universal immunization of all infants with nirsevimab is expected to reduce 290 174 RSV-MALRTI, 24 986 hospitalizations, and expenditures of $612 million 2021 USD.
Conclusions
An all-infant immunization strategy with nirsevimab could substantially reduce the health and economic burden for US infants during their first RSV season. While this reduction is driven by term infants, all infants, including palivizumab-eligible and preterm infants, would benefit from this strategy.
Keywords: economics, model, lower respiratory tract illness, nirsevimab, RSV, burden, cost, infants, United States
Respiratory syncytial virus (RSV) infection is the main cause of severe lower respiratory tract illness (LRTI) in infants; approximately 90% of children younger than 2 years of age become infected, accounting for up to 40% of pediatric cases of pneumonia and 72% of cases of acute bronchiolitis requiring hospitalization [1–4]. In the United States, RSV is a leading cause of infant hospitalization, with an overall RSV-related hospitalization rate in infants of 0.8%–2.6% [5–7], and mortality rate of 9 per 10 000 admissions [8]. RSV is a seasonal virus, with the initiation and duration of circulation largely dependent on geographical location [9].
While preterm infants, especially those with chronic health conditions, have a greater risk for medically attended RSV-associated LRTIs (RSV-MALRTI) and the highest morbidity and mortality rates among infants [10], 72% of infants hospitalized with RSV are healthy term infants without underlying health conditions [5, 11, 12]. Palivizumab, a monoclonal antibody (mAb), is currently the only available option in the United States to prevent RSV and is only recommended for a small subset of infants <2 years of age with hemodynamically significant congenital heart disease (CHD), chronic lung disease of prematurity (CLDP), and infants born prematurely at <29 weeks’ gestational age (wGA) [13–16]. Palivizumab requires monthly administrations throughout the RSV season, resulting in both logistical and financial barriers for uptake [14]. There is currently no approved or recommended prophylaxis option for healthy term and preterm infants born at ≥29 wGA, reflecting a need for broad measures that can protect infants across all gestational ages and risk factors.
Nirsevimab is a long-acting mAb being investigated for the prevention of RSV-MALRTI in infants [17]. In term and preterm infants, nirsevimab demonstrated an overall efficacy of 79.5% (95% confidence interval [CI], 65.9%–87.7%) in the prevention of RSV-MALRTI in prespecified, pooled analyses of the pivotal phase 2b (NCT02878330) and phase 3 MELODY (NCT03979313) studies [18–20]. Previous modelling studies have evaluated the impact of prophylactic measures on the RSV disease burden [21–24]; however, none have explicitly compared nirsevimab against the current standard of care (SoC) using the clinical trial efficacy results. Therefore, the objective of this study was to evaluate the health and cost outcomes associated with the use of nirsevimab against SoC in the prevention of RSV-MALRTIs in all infants in their first RSV season in the United States.
METHODS
Target Population and Immunization Strategies
The entire US birth cohort served as the basis of the model, and infants were stratified into subpopulations to account for the differential individual risks of severe RSV-MALRTI. The subpopulations corresponded to the 3 groups assessed in the nirsevimab clinical trials [18–20]: (1) term and late preterm infants (born at or after 35 wGA); (2) preterm infants (born between 29 wGA and 34 weeks, 6 days GA, not eligible for palivizumab [13]); and (3) palivizumab-eligible infants (infants born before 29 wGA or those with CLDP or CHD per the latest American Academy of Pediatrics recommendations [13]) (Table 1).
Table 1.
Model Inputs
| Input | Palivizumab Eligible Infants | Preterm Infants | Term Infants |
|---|---|---|---|
| Population size, %a | 1.6 [29, IQVIA, unpublished data] | 4.2 [31] | 94.2 |
| Palivizumab product profile | |||
| Efficacy, % | 51b [32] | NA | NA |
| Time to onset of protection | Immediate | NA | NA |
| Uptake, % | 58 [IQVIA, unpublished data, Sanofi, unpublished data] | NA | NA |
| Duration of protection by dose, mo | 1 | NA | NA |
| Nirsevimab product profile | |||
| Efficacy, % | Noninferior | 79.5 [18] | 79.5 [18] |
| Time to onset of protection | Immediate | Immediate | Immediate |
| Uptake, % | 80 [Sanofi, unpublished data] | 71 [Sanofi, unpublished data] | 71 [Sanofi, unpublished data] |
| Duration of protection by dose, mo | 5 | 5 | 5 |
| Hospitalization rates, % | Hospitalizations [25, 33] | Hospitalizations [28] | Hospitalizations [28] |
| Raw rate from source | … | 2.2 | 1.3 |
| Raw rate for infants with CHD [25, 33] | 9.7 | … | … |
| Raw rate for infants with CLD [25, 33] | 12.8 | … | … |
| Raw rate for infants <29 wGA [25, 33] | 8.1 | … | … |
| Per-inpatient risk, %, ICU, MV [5] | |||
| 0–2 mo | 50, 17 | 62, 23 | 31, 9 |
| 3–5 mo | 29, 5 | 27, 6 | 23, 2 |
| 6–11 mo | 19, 5 | 18, 5 | 17, 2 |
| Outpatient rates for all infants, %, ER, PC [26]c | |||
| 0 mo | 2.0, 8.5 | ||
| 1 mo | 6.4, 18.8 | ||
| 2 mo | 7.2, 23.4 | ||
| 3 mo | 10.5, 23.3 | ||
| 4 mo | 11.6, 26.5 | ||
| 5 mo | 7.1, 28.9 | ||
| 6 mo | 8.2, 26.5 | ||
| 7 mo | 5.6, 20.7 | ||
| 8 mo | 5.6, 27.8 | ||
| 9 mo | 5.6, 22.7 | ||
| 10 mo | 4.0, 24.2 | ||
| 11 mo | 5.6, 25.8 | ||
| Proportion of LRTI among health events for all infants, %, hospitalizations, ER visits, PC visits [30] | |||
| 0–5 mo | 100, 65, 65 | ||
| 6–11 mo | 100, 50, 30 | ||
| Mortality rates for all infants, %, All-cause mortality [5, 34], RSV-related mortality [27]c | |||
| 0–11 mo | 0.05, 0.0024 | ||
| Cost by event, 2021 USD | |||
| Inpatient hospitalization [28] | $38 626 | $16 131 | $9250 |
| ICU [28] | $66 031 | $46 823 | $34 362 |
| MV [28] | $122 366 | $81 199 | $77 855 |
| ER visits [35] | $501 | $501 | $501 |
| PC visits [36, 37] | $118 | $118 | $118 |
RSV season is from October to March [21].
Abbreviations: CHD, congenital heart disease; CLD, chronic lung disease; ER, emergency room; ICU, intensive care unit; MV, mechanical ventilation; NA, not applicable; PC, primary care; RSV, respiratory syncytial virus; wGA, weeks’ gestational age.
Based on 3 711 000 annual live births [31].
Assumed noninferiority between nirsevimab and palivizumab.
Due to data limitations, data stratified by subpopulation were unavailable. As a result, the same inputs are applied equally for all subpopulations in the analysis.
The model compared 2 immunization strategies: (1) the current SoC for each subpopulation, consisting of up to 5 monthly administrations of palivizumab during the RSV season for eligible infants only, and no prophylaxis for other term and preterm infants; and (2) passive immunization with nirsevimab for all infants (Supplementary Figure 1). Both strategies employed a seasonal approach whereby prophylaxis was administered only at the beginning of or during the RSV season for all infants younger than 1 year in their first RSV season. In strategy 1, the first dose of palivizumab was administered at the start of the season (ie, October) for eligible infants born outside the RSV season (March–September) or at birth for those born within the RSV season (October–February). In strategy 2, a single dose of nirsevimab was administered at the start of the RSV season for infants born out of season and at birth for those born within season. Infants born in March were considered as born out of season due to the low circulation level of RSV.
Model Overview
A static decision analytic model was developed in Microsoft Excel that tracks the US birth cohort during its first RSV season and considers the possible RSV-related health events and their associated costs in this population (Supplementary Figure 2). All infants in the model were considered susceptible to RSV-MALRTIs, with risk changing during the year, depending on age, density of the RSV circulation over the season, and infant subpopulation. The RSV season was defined as a 6-month period—October to March, with a peak in December [21]—based on data from the Centers for Disease Control and Prevention (CDC) describing the distribution of the RSV cases across the year. No circulation of the virus was considered outside of the 6-month season [21] (Supplementary Figure 3).
The estimate of RSV-MALRTI during the first RSV season of infants was defined based on the combination of 3 components: (1) proportion of RSV cases for a given month, (2) incidence rate per month of age, and (3) age at start of the season (Figure 1). These estimates were then multiplied by a clinical severity factor determining the proportion RSV-MALRTI among all RSV cases [1].
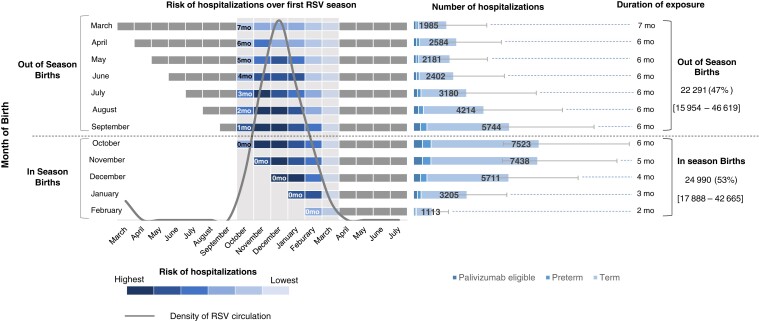
Figure 1.
Risk and number of RSV-LRTI hospitalizations of US infants in their first respiratory syncytial virus season, by month of birth. Total values indicate number and percentage of RSV-LRTI hospitalizations occuring in infants born in season (October to February) and out of season (March to September). Vertical gray bars represent RSV season (October to March), error bars and values in square brackets reflect the uncertainty in RSV-LRTI hospitalizations associated with uncertainty in RSV rates. Abbreviations: mo, month; LRTI, lower respiratory tract illness; RSV, respiratory syncytial virus.
The impact of prophylactic measures was based on the intervention's uptake (coverage rate) and efficacy, defined as reduction of RSV-MALRTIs, to estimate the total number of events in each strategy (Table 1). An immediate onset of protection and a 5-month duration of protection with no residual efficacy after was assumed.
The health events included inpatient hospitalizations, intensive care unit (ICU) admissions, mechanical ventilation (MV), emergency room (ER) visits, and primary care visits. The model also accounts for all-cause infant mortality by age, applied to the unadjusted birth cohort before determining the estimated RSV cases, as well as the risk of death among infants with RSV.
The results for each strategy and incremental impact were presented from a full birth cohort perspective, with detailed results based on health events and related costs (1) per risk group (term, preterm, and palivizumab eligible); and (2) per chronological age at the start of the season. Multiway deterministic sensitivity analyses were performed to test the key model drivers and determine the robustness of model conclusions accounting for uncertainty in model parameters.
Model Inputs
The proportion of infants eligible for palivizumab was determined as the sum of the percentages of infants who were born <29 wGA preterm, with CHD and CLDP. The percentage of preterm infants (not eligible for palivizumab) was estimated based on the number of births between 29 wGA and 34 weeks, 6 days GA, less the number of births having comorbid conditions specified for the palivizumab-eligible population. Inputs regarding the per-patient risk of RSV-related health events were stratified by subpopulation and per month of age for the first year (0 to 11 months).
Inpatient hospitalization rates for the palivizumab-eligible population prior to the introduction of palivizumab were informed by Feltes and Simoes [25] for infants with CHD and by the IMpact-RSV study group [33] for infants with CLDP and those born <29 wGA. The McLaurin study [28] informed the hospitalization rates among preterm and term infants. Transformation of these overall estimates into estimates by month of age was based on the monthly trend derived from Hall et al [6]. Additionally, 100% of inpatient hospitalizations for all ages were assumed to be LRTIs [21].
The rates for ER and primary care visits were informed by Lively et al [26], while the proportion of hospital admissions requiring ICU admission or MV was informed by Arriola et al [5]. A total of 65% of ER visits were assumed to be LRTIs for the first 5 months of age followed by 50% starting in month 6 [21]. Similarly, 65% of primary care visits were assumed to be LRTIs in the first 5 months of age followed by 30% starting at 6 months [21]. The remaining cases were considered as upper respiratory infections, for which nirsevimab is not expected to have an impact per clinical trial end points [21]. Respiratory deaths for infants ages 0–11 months presented in Hansen et al [27] were used to inform RSV-related mortality.
To account for the uncertainty around incidence rates of health events, lowest and highest uncertainty ranges were used when available. Hall et al [6] and Stockman et al [7] were used as lower and higher bounds for hospitalizations rates for term infants, pneumonia and influenza death rates as lower bound and respiratory and circulatory death rates as higher bound for RSV-related mortality [27], and 95% CIs as reported in the original studies for ICU admission rates (among term infants) [5], ER, and primary care visits (for all subpopulations) [26].
The overall pooled efficacy of nirsevimab in the prevention of RSV-MALRTIs was utilized for all term and preterm infants, and noninferiority in terms of protection against RSV-MALRTIs versus palivizumab was assumed for the palivizumab-eligible population, according to MEDLEY, head-to-head phase 2/3 trial of nirsevimab versus palivizumab [38]. A comprehensive list of model parameters is presented in Table 1, and detailed calculations and final parameters per subpopulation and age in months is presented in Supplementary Table 1.
RESULTS
Disease Burden Under the Current SoC
The burden of RSV-MALRTIs of the US birth cohort in their first RSV season was estimated to be 529 915 cases (range, based on uncertainty of model inputs: 419 145–649 260), affecting 14% of this population. An estimated 67% (353 563; range: 294 784–412 313) of RSV-MALRTIs led to a primary care visit, 24% (129 070; range: 110 519–147 663) required ER visits, and 9% (47 281; range: 33 842–89 284) resulted in hospitalizations (Supplementary Figure 4A).
Of the total hospitalizations, 28% (13 411; range: 6789–34 607) were treated in the ICU, while 7% (3257; range: 2472–5393) required MV. The model also estimated 75 RSV-related deaths. The estimated overall direct economic burden of RSV was $1.2 billion (range: $881 million to $2.3 billion) 2021 USD. Although hospitalized cases (including ICU admissions and MV) made up less than 10% of the overall RSV-MALRTIs burden, their associated costs represented 91% ($1.1 billion; range: $791 million–$2.2 billion) of the total direct medical costs annually (Supplementary Figure 4B and Supplementary Table 2).
During the first RSV season, approximately 93% of RSV-related hospitalizations (28 909; range: 22 877–47 580) occurred in infants who are currently not eligible to receive prophylaxis, while these same infants accounted for up to 82% of the total hospitalization costs including ICU admissions and MV ($934 million; range: $590 million–$2.0 billion) (Supplementary Figure 5A and Supplementary Figure 5B). The distribution of RSV-LRTI hospitalizations during the first RSV season was relatively balanced between infants born in season (53%, 24 990 RSV-LRTI hospitalizations; range: 17 888–42 665) and out of season (47%, 22 920 RSV-LRTI hospitalizations; range: 15 953–46 618) (Figure 1 and Supplementary Table 4). When accounting for all RSV-MALRTIs during the first RSV season, 69% of the overall health events (364 564; range: 303 459–443 706) and 43% of related costs ($529 million; range: 373 million–$1.1 billion) were attributed to infants born out of season, and 31% of health events (165 351; range: 135 685–205 553) and 57% of related costs ($713 million; range: $509 million–$1.2 billion) were attributed to infants born in season (Figure 2 and Supplementary Table 5).
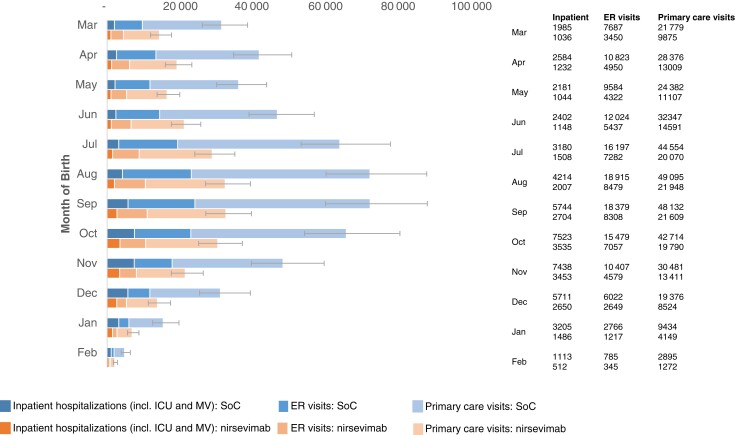
Figure 2.
Respiratory syncytial virus-related health events of US infants in their first respiratory syncytial virus season under current standard of care or universal immunization with nirsevimab, by month of birth. Error bars reflect the uncertainty in RSV-MALRTIs associated with uncertainty in RSV rates. Abbreviations: ER, emergency room; ICU, intensive care unit; MV, mechanical ventilation; SoC, standard of care.
Disease Burden Under All Infant Immunization With Nirsevimab
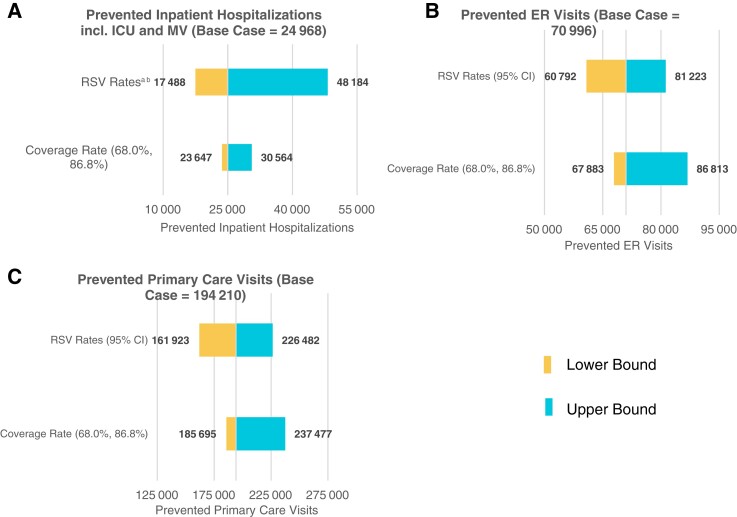
Based on 71% and 80% uptake rates in term/preterm infants and palivizumab-eligible infants, respectively, the use of nirsevimab resulted in 290 174 (range: 240 202–355 890) fewer RSV-MALRTIs in the annual US birth cohort, and 55% reduction in health events compared to SoC. Universal immunization with nirsevimab prevented 24 986 (range: 17 487–48 185) hospital admissions (53% reduction; range: ), 70 996 (range: 60 792–81 223) ER visits (55% reduction), and 194 210 (range: 161 923–226 482) primary care visits (55% reduction) (Supplementary Table 2). Hospitalized patients saw a 52% reduction (vs SoC) in ICU admissions (6963 fewer cases; range: 3274–18 709) and a 51% reduction in MV (1664 fewer cases; range: 1225–2850) relative to SoC. The use of nirsevimab reduced direct medical costs by 49%—an annual savings of $612 million (range: $411 million–$1.2 billion) 2021 USD (Supplementary Table 3).
As most RSV-MALRTIs occur among term infants, 95% of the RSV-MALRTI encounters prevented by an all-infant immunization strategy (290 174 RSV-MALRTIs; range: 240 203–355 890) were in this group (Supplementary Figure 6). 91% of prevented hospitalizations (35 606; range: 15 139–45 835), 95% of prevented ER (7 727; range: 57 992–77 483) and primary care visits (185 268; range: 154 468–216 054) were also among term infants, as were 83% of medical costs ($505 million; range: $305 million–$1.1 billion) (Supplementary Figure 7). An all-infant immunization strategy would also prevent a disproportionately high number of RSV-MALRTIs among preterm and palivizumab-eligible infants, small populations with high rates of RSV-MALRTIs. The impact of this strategy among infants born prior to the start of the RSV season is the prevention of 199 451 RSV-MALRTIs (range: 165 910–242 881) and $257 million 2021 USD (range: $171 million–$570 million) in RSV-related costs, while 90 724 cases (range: 74 290–113 009) and $355 million in costs (range: $240 million–$642 million) can be prevented in infants born within the RSV season (Table 2). Sensitivity analysis on coverage rates and incidence rates are presented in Figure 3 and Figure 4. Supplementary Figure 8 presents the sensitivity analysis around efficacy of nirsevimab based on phase 2B and phase 3 clinical trial end points.
Table 2.
Prevented RSV-Related Health Events and Related Direct Medical Costs, by Month of Birth and Age at the Start of the Season
| Month of Birth (Age at Start of RSV Season) |
Prevented Health Events | Prevented Medical Costs in 2021 USDa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hospitalizationsb | ICUb | MV | ER Visits | PC Visits | Hospitalizations | ICUc | MVc | ER Visits | PC Visits | |
| March (7 mos) | 765 | 162 | 22 | 4237 | 11 904 | $7 889 085 | $5 856 728 | $1 740 855 | $2 123 781 | $1 402 680 |
| April (6 mos) | 1090 | 231 | 31 | 5873 | 15 367 | $11 239 765 | $8 344 218 | $2 480 238 | $2 943 655 | $1 810 701 |
| May (5 mos) | 910 | 201 | 26 | 5261 | 13 275 | $9 380 592 | $7 246 483 | $2 091 229 | $2 636 847 | $1 564 190 |
| June (4 mos) | 981 | 245 | 29 | 6588 | 17 756 | $10 091 371 | $8 839 010 | $2 328 171 | $3 301 665 | $2 092 178 |
| July (3 mos) | 1269 | 364 | 39 | 8915 | 24 484 | $13 033 543 | $13 186 080 | $3 140 097 | $4 468 199 | $2 884 987 |
| August (2 mos) | 1590 | 541 | 76 | 10 436 | 27 146 | $16 216 007 | $19 662 694 | $6 137 660 | $5 230 596 | $3 198 627 |
| September (1 mo) | 1996 | 860 | 185 | 10 071 | 26 523 | $20 050 877 | $31 533 918 | $14 797 198 | $5 047 542 | $3 125 157 |
| October (0 mo) | 2399 | 1252 | 337 | 8422 | 22 924 | $23 712 486 | $46 169 128 | $26 983 937 | $4 221 240 | $2 701 150 |
| November (0 mo) | 2305 | 1303 | 377 | 5828 | 17 070 | $22 682 577 | $48 184 940 | $30 145 277 | $2 921 243 | $2 011 345 |
| December (0 mo) | 1735 | 1021 | 305 | 3373 | 10 852 | $17 026 649 | $37 806 505 | $24 393 357 | $1 690 584 | $1 278 681 |
| January (0 mo) | 965 | 579 | 175 | 1551 | 5285 | $9 467 744 | $21 543 704 | $14 080 248 | $777 170 | $622 768 |
| February (0 mo) | 335 | 204 | 62 | 441 | 1623 | $3 374 983 | $7 715 233 | $5 035 554 | $220 784 | $191 235 |
| Total (lower–upper bound range) | 16 341 (12 988–26 626) | 6963 (3274–18 709) | 1664 (1225–2850) | 70 996 (60 792–81 223) | 194 210 (161 923–226 482) | $164 165 679 ($133 156 005–$259 297 239) | $256 088 641 ($129 344 792–$659 696 379) | $133 353 821 ($99 204 252–$225 680 968) | $35 583 305 ($30 468 741–$40 709 189) | $22 883 699 ($19 079 330–$26 686 236) |
Results of the analysis presented in this table are generated based on the input parameters presented within Table 1.
Abbreviations: ER, emergency room; ICU, intensive care unit; MV, mechanical ventilation; PC, primary care.
All costs are in 2021 US dollars.
Case counts presented here are disaggregated estimates (ie, hospitalizations are hospitalizations alone while ICU admissions are hospitalizations that led to an ICU admission alone without MV).
Although case counts for these resources are presented in a disaggregated manner, input parameters for the costs associated with ICU admissions and MV are inclusive (eg, the cost of ICU admission includes the cost of initial hospitalization and ICU admission while the cost of MV incorporates the cost of initial hospitalization, ICU admission, and resulting MV.
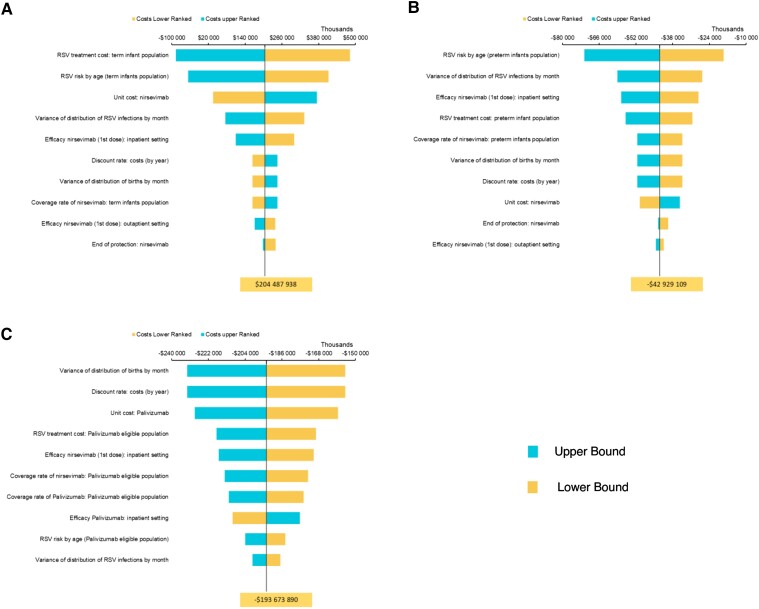
Figure 3.
Deterministic sensitivity analysis tornado diagrams of prevented respiratory syncytial virus-related health events with nirsevimab immunization all US infants in their first RSV season, by event type: (A) inpatient hospitalizations (including ICU admissions and MV), (B) emergency room visits, and (C) primary care visits. aLB (for term infants only): hospitalizations, Hall et al [6]; ICU admissions, LB of 95% CI; MV, 20% reduction in risk of MV. bUB (for term infants only): hospitalization, Stockman et al [7]; ICU admissions, UB of 95% CI; MV, 20% increase in risk of MV. Abbreviations: CI, confidence interval; ER, emergency room; ICU, intensive care unit; LB, lower bound; MV, mechanical ventilation; RSV, respiratory syncytial virus; UB, upper bound.
Figure 4.
Deterministic sensitivity analysis tornado diagrams of incremental costs (in 2021 USD) with nirsevimab immunization all US infants in their first RSV season, by population subgroup: (A) term infants, (B) preterm infants, and (C) palivizumab-eligible infants. Abbreviation: RSV, respiratory syncytial virus.
DISCUSSION
This was the first study to describe the expected impact of universal infant immunization with nirsevimab on RSV-related health outcomes and expenditures. Infants ineligible for palivizumab—term and preterm—accounted for 93% of the hospitalization burden, despite having a lower risk of severe events. This highlighted a scale effect, that even though premature infants with CHD and CLD are at greatest risk of serious outcomes from RSV infections, the immunization of term infants (accounting for approximately 98% of the birth cohort) would prevent the greatest amount of health outcomes and expenditures.
The results of this model suggested that the use of nirsevimab in all infants could reduce health events by 55% and the overall costs to the payer by 49%. Immunizing infants born outside of season would prevent 199 451 total RSV-LRTIs and avoid $257million (2021 USD) of direct medical costs. For infants born within season, an estimated 90 724 RSV-LRTIs would be avoided, resulting in $355million (2021 USD) in savings. Over two-thirds of RSV-MALRTI occurred in infants born outside the season, driven by a peak of outpatient visits later in the first year of life, as previously observed [26]. Despite lower hospitalization rates, the number of hospitalizations in the oldest age groups (ie, infants born outside the season) was almost equivalent to those in the youngest infants born within the season. This modelled RSV hospitalization distribution pattern was validated through 2 real-world studies in France and the United Kingdom [39, 40]. The RSV burden in infants born outside the season can be explained by a longer duration of exposure to RSV. Those infants experience a full RSV season compared to infants born within the season (particularly those born after the RSV peak), who despite their increased vulnerability have less exposure to the virus.
The seasonality of RSV informs a critical component of the immunization strategy, and the results of this study suggest that the optimal immunization timing for infants born outside the season would be at the start of the RSV season in October (for infants up to 7 months of age) to maximize their protection for the entire RSV season. This strategy allows the immunization of infants born outside the season at the start of the epidemic to prevent half of the hospitalized cases of RSV, and infants born within the season at birth to protect the youngest and more vulnerable infants. Compared to other prophylactic strategies, the use of nirsevimab allows for the protection of all infants during their window of vulnerability, due to the ability of nirsevimab to be administered at any time with a rapid onset of protection.
The key model strength was the ability to stratify estimates by age at the time of infection and by infant subpopulation, thus expanding on the design of previous models [21]. Hospitalization rates by wGA from McLaurin et al [28] allowed for the consideration of the risk by subpopulation and the respective benefit of nirsevimab based on the clinical trial results. Additionally, by stratifying the analyses by age at the time of infection, the model could evaluate the potential impact of nirsevimab on infants born within versus outside the season. The potential to disaggregate the population at the finest level (ie, by subpopulation, age in month, calendar month) allowed optimal strategies to be assessed and evidence to be obtained on the most impactful public health outcome to inform the decision-making process. The model also included the risk of ICU admissions and MV for inpatient admissions, the impact of which has not been studied by prior models.
Overall, the current model aligned with recent studies evaluating the impact of immunizations on RSV-MALRTIs [21, 22, 24, 41]. Rainisch et al [21] used a static impact model to assess the impact of palivizumab, mAbs, and maternal vaccines on the incidence of RSV-related health care outcomes [21]. Although the current model was structured with a more detailed approach on infant subpopulations, considering differentiated inputs and efficacy for each, the overall results showed that nirsevimab is an effective alternative to palivizumab, with a similar reduction of cases across the birth cohort.
The current analysis has several limitations, largely due to a lack of robust data. The rate of RSV-related hospitalizations among term infants was a key driver of the model, and this study used as a base case the rates from McLaurin et al, a retrospective analysis of infants younger than 1 year based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes for their hospitalizations [28]. However, the available evidence for RSV-related hospitalizations in the United States varied substantially, with Hall et al [6] providing the lowest rates, and Stockman et al [7] reporting the highest. While Hall only included laboratory-confirmed RSV acute respiratory illness, Stockman used ICD-9-CM codes and included a proportion of hospitalizations coded as bronchiolitis and pneumonia, which may have contributed to the difference in the hospitalization rates between the 2 studies. To test the impact of the variability in hospitalization rates, we constructed the uncertainty range around the RSV-related hospitalization rate among term infants, and ran analyses using estimates from Hall and Stockman as the lower and higher uncertainty range.
Another limitation, given the model structure, was the need for more granular data, which sometimes led to data gaps. In these situations, assumptions were made to fit the available data. For example, rates for ER and primary care visits were assumed to be consistent across all risk groups. Estimates for inpatient hospitalizations were also not always available by the month of age, and therefore the monthly trend for the overall population from Hall et al [6] was assumed to be applicable to all subpopulations. Finally, for alignment and comparison with the analysis performed by Rainisch et al [21], we applied the same assumptions for the proportion of LRTIs in health events, which cites unpublished data from the CDC, and further information would be needed.
The differences in the reduction of outcomes with the use of nirsevimab by population were due to the differential risk of RSV-MALRTI and specific coverage rates. For palivizumab-eligible infants, while a noninferior efficacy was considered for nirsevimab versus palivizumab, the model assumed higher uptake rate of nirsevimab in the palivizumab-eligible population, due to likely better compliance with a single dose of nirsevimab versus monthly injection of palivizumab. For preterm and term infants ineligible for palivizumab, a consistent prespecified pooled efficacy was applied across all infants. Indeed, due to the mechanism of action and pharmacokinetics of nirsevimab, there is no basis by which to expect differential efficacy in the various infants’ subpopulations. This has been borne out in the results of the pivotal studies of nirsevimab, designed to encompass all infants, where consistent levels of efficacy were demonstrated in preterm versus term subgroups, and across the spectrum of disease severity.
Furthermore, mid- to long-term sequelae following RSV-MALRTIs were not considered in the present analysis. However, using similar assumptions for wheezing as in a recent cost-effectiveness analysis on the prevention of RSV cases in Norway, we conducted scenario analyses which estimated the prevention of over 18 000 cases of wheezing over 3 years with nirsevimab (Supplementary Table 5).
As a conservative approach, the model did not consider the effect of waning immunity of nirsevimab after 5 months of protection. However, given that the analyses were performed for a single RSV season, waning would only affect the final month for infants born outside of the season and was not expected to impact the study conclusions. Assumptions on the RSV seasonality in the United States was also based on historic national averages and may not necessarily reflect the geographical variation in the onset and offset of the RSV season in areas such as southeastern United States (eg, Florida). The implementation of an infant nirsevimab program will likely vary geographically to best reflect the seasonality of RSV to ensure infants are protected during the peak of the RSV season.
Costs for inpatient hospitalization, ICU, and MV reported in McLaurin et al [28] were based on the total claim amount, including insurance and patient out-of-pocket payments, assumed to be 20% of the total claim, although the actual cost to the payer could vary. Furthermore, the analysis focuses on direct costs only and the costs associated with purchasing and administering nirsevimab are not included in this study.
Finally, given that this is a static model, potential indirect effects have not been captured. The potential effects of RSV antibodies on the infectiousness of the virus and the susceptibility of infants and children to infection will need to be further explored to refine assumptions on mechanism of action of passive immunization with monoclonal antibodies.
CONCLUSION
An all-infant immunization strategy with nirsevimab compared to the current SoC in the United States could substantially reduce the health and economic burden associated with infants during their first RSV-LRTI season. While this reduction would be driven by term infants, who account for most of the RSV-MALRTI burden, all infants, including palivizumab-eligible and preterm infants who suffer from significantly higher rates of disease, would benefit from this immunization strategy, addressing the public health and clinical aims of reducing RSV-MALRTI burden and protecting all infants.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Deuk Kang, PharmD for assistance with manuscript preparation; and Christian Felter, MD, Michael E. Greenberg, MD MPH, Christopher B. Nelson, PhD MPH, and Ayanna Santos, PharmD for critically reviewing the manuscript.
Financial support. This work was supported by AstraZeneca and Sanofi.
Supplement sponsorship. This article appears as part of the supplement “Respiratory Syncytial Virus Disease Among US Infants,” sponsored by Sanofi and AstraZeneca.
Supplementary Material
Contributor Information
Alexia Kieffer, Sanofi, Lyon, France.
Matthieu Beuvelet, Sanofi, Lyon, France.
Aditya Sardesai, Evidera Inc, San Francisco, California, USA.
Robert Musci, Evidera Inc, San Francisco, California, USA.
Sandra Milev, Evidera Inc, San Francisco, California, USA.
Julie Roiz, Evidera Inc, London, UK.
Jason K H Lee, Sanofi, Toronto, Ontario, Canada.
References
- 1. Shi N, Palmer L, Chu BC, et al. Association of RSV lower respiratory tract infection and subsequent healthcare use and costs: a Medicaid claims analysis in early-preterm, late-preterm, and full-term infants. J Med Econ 2011; 14:335–40. [DOI] [PubMed] [Google Scholar]
- 2. Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med 2012; 166:700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008; 86:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greenough A, Cox S, Alexander J, et al. Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Arch Dis Child 2001; 85:463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arriola CS, Kim L, Langley G, et al. Estimated burden of community-onset respiratory syncytial virus-associated hospitalizations among children aged<2 years in the United States, 2014–15. J Pediatric Infect Dis Soc 2020; 9:587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013; 132:e341–8. [DOI] [PubMed] [Google Scholar]
- 7. Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatr Infect Dis J 2012; 31:5–9. [DOI] [PubMed] [Google Scholar]
- 8. Byington CL, Wilkes J, Korgenski K, Sheng X. Respiratory syncytial virus-associated mortality in hospitalized infants and young children. Pediatrics 2015; 135:e24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health 2019; 7:e1031–45. [DOI] [PubMed] [Google Scholar]
- 10. Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35:519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rha B, Curns AT, Lively JY, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015-2016. Pediatrics 2020; 146:e20193611. [DOI] [PubMed] [Google Scholar]
- 12. Barr R, Green CA, Sande CJ, Drysdale SB. Respiratory syncytial virus: diagnosis, prevention and management. Ther Adv Infect Dis 2019; 6:2049936119865798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee . Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134:415–20. [DOI] [PubMed] [Google Scholar]
- 14. Sobi Inc. Synagis (palivizumab) prescribing information (revised November 2021). https://www.synagis.com/synagis.pdf. Accessed 11 May 2022.
- 15. Oymar K, Skjerven HO, Mikalsen IB. Acute bronchiolitis in infants, a review. Scand J Trauma Resusc Emerg Med 2014; 22:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Breakell R, Thorndyke B, Clennett J, Harkensee C. Reducing unnecessary chest X-rays, antibiotics and bronchodilators through implementation of the NICE bronchiolitis guideline. Eur J Pediatr 2018; 177:47–51. [DOI] [PubMed] [Google Scholar]
- 17. Domachowske JB, Khan AA, Esser MT, et al. Safety, tolerability and pharmacokinetics of MEDI8897, an extended half-life single-dose respiratory syncytial virus prefusion F-targeting monoclonal antibody administered as a single dose to healthy preterm infants. Pediatr Infect Dis J 2018; 37:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med 2022; 386:837–46. [DOI] [PubMed] [Google Scholar]
- 19. Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020; 383:415–25. [DOI] [PubMed] [Google Scholar]
- 20. Simões E, Madhi SA, ZAR HJ, et al. Pooled efficacy of nirsevimab against RSV lower respiratory tract infection in preterm and term infants. 40th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID 2022).Athens, Greece, May 9–13, 2022. [Google Scholar]
- 21. Rainisch G, Adhikari B, Meltzer MI, Langley G. Estimating the impact of multiple immunization products on medically-attended respiratory syncytial virus (RSV) infections in infants. Vaccine 2020; 38:251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hodgson D, Pebody R, Panovska-Griffiths J, Baguelin M, Atkins KE. Evaluating the next generation of RSV intervention strategies: a mathematical modelling study and cost-effectiveness analysis. BMC Med 2020; 18:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li X, Willem L, Antillon M, Bilcke J, Jit M, Beutels P. Health and economic burden of respiratory syncytial virus (RSV) disease and the cost-effectiveness of potential interventions against RSV among children under 5 years in 72 Gavi-eligible countries. BMC Med 2020; 18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cromer D, van Hoek AJ, Newall AT, Pollard AJ, Jit M. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: a modelling and cost-effectiveness analysis for England. Lancet Public Health 2017; 2:e367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feltes TF, Simoes E. Palivizumab prophylaxis in haemodynamically significant congenital heart disease. Arch Dis Child 2005; 90:875–7; author reply 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lively JY, Curns AT, Weinberg GA, et al. Respiratory syncytial virus-associated outpatient visits among children younger than 24 months. J Pediatric Infect Dis Soc 2019; 8:284–6. [DOI] [PubMed] [Google Scholar]
- 27. Hansen CL, Chaves SS, Demont C, Viboud C. Mortality associated with influenza and respiratory syncytial virus in the US, 1999-2018. JAMA Netw Open 2022; 5:e220527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol 2016; 36:990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pavilack M, Clifford RA, Gonzales T, Kong AM, Wade S, McLaurin KK. Trends in utilization of outpatient respiratory syncytial virus prophylaxis with palivizumab among medicaid- and commercially insured infants. Infect Dis Ther 2018; 7:121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019; 7:e37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Center for Disease Control and Prevention . National vital statistics system: state and national provisional counts. https://www.cdc.gov/nchs/nvss/vsrr/provisional-tables.htm. Accessed 1 October 2021.
- 32. Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev 2013; (4):CD006602. [DOI] [PubMed] [Google Scholar]
- 33. The IMpact-RSV Study Group . Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531–7. [PubMed] [Google Scholar]
- 34. National Vital Statistics System, Center for Disease Control and Prevention . Deaths: final data for 2015. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_06.pdf. Accessed 1 October 2021. [PubMed]
- 35. Agency for Healthcare Research and Quality . Healthcare cost and utilization project: costs of emergency department visits in the United States, 2017. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb268-ED-Costs-2017.pdf. Accessed 1 October 2021. [PubMed]
- 36. InHealth Professional Services. Physicians’ Fee and Coding Guide (Payment Range). [Google Scholar]
- 37. Centers for Medicare and Medicaid Services . Physician fee schedule look-up tool. National 2021 payment amount by HCPCS code. www.cms.gov. Accessed 1 October 2021.
- 38. Domachowske J, Madhi SA, Simões EA, et al. Safety of nirsevimab for RSV in infants with heart or lung disease or prematurity. N Engl J Med 2022; 386:892–4. [DOI] [PubMed] [Google Scholar]
- 39. Demont C, Petrica N, Bardoulat I, et al. Economic and disease burden of RSV-associated hospitalizations in young children in France, from 2010 through 2018. BMC Infect Dis 2021; 21:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reeves RM, Hardelid P, Panagiotopoulos N, Minaji M, Warburton F, Pebody R. Burden of hospital admissions caused by respiratory syncytial virus (RSV) in infants in England: a data linkage modelling study. J Infect 2019; 78:468–75. [DOI] [PubMed] [Google Scholar]
- 41. Regnier SA. Respiratory syncytial virus immunization program for the United States: impact of performance determinants of a theoretical vaccine. Vaccine 2013; 31:4347–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.