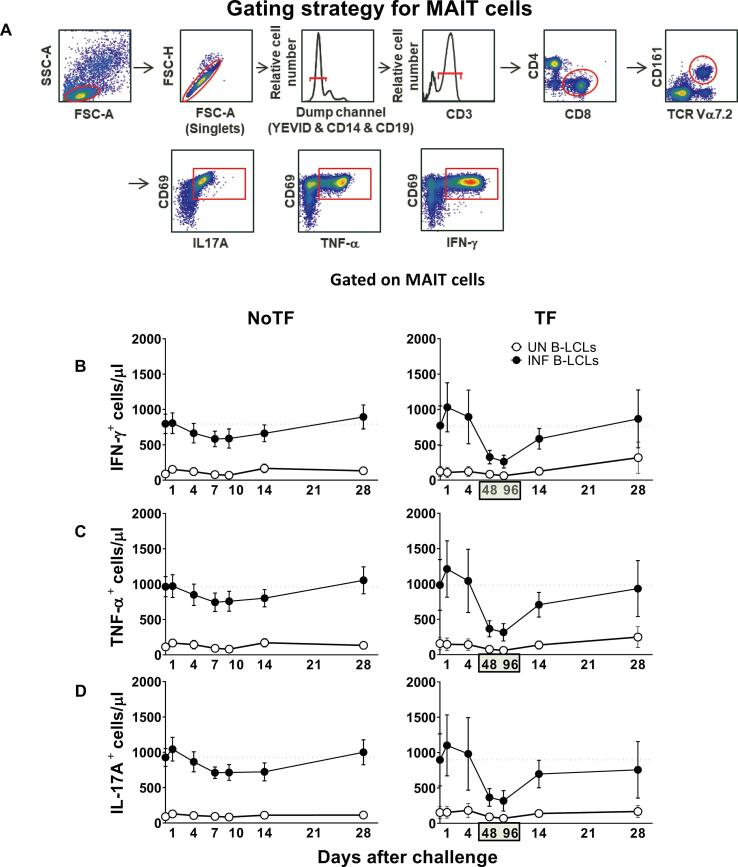
Fig. 1.
Kinetics of cytokine-producing MAIT cells from participants over a 28-day post-challenge follow-up period.Ex vivo PBMC from participants receiving a S. Typhi inoculum were stained with YEVID, followed by surface staining with mAbs to CD3, CD4, CD8, CD14, CD19, CD161, and TCRα 7.2. After fixation and permeabilization, cells were intracellularly stained with monoclonal antibodies to CD69, as well as to IFN-γ, TNF-αα, and IL-17A cytokines and analyzed by flow cytometry. For the analysis, following the elimination of doublets and other debris, a “dump” channel was used to eliminate dead cells (YEVID+) as well as macrophages (CD14+), and B cells (CD19+) from the analyses. This was followed by additional gating on CD3, CD4, and CD8, as well as CD161 versus TCRα 7.2 to analyze MAIT cells, and afterward on CD69, IFN-γ, TNF-α, and IL-17A to evaluate cytokine secretion. (A) Representative gating strategy for MAIT cells. Kinetics of the production of (B) IFN-γ, (C) TNF-α, and (D) IL-17A by MAIT cells following exposure to (●) autologous B-LCLs infected with S. Typhi (INF B-LCLs), or controls (○) (UN, uninfected B-LCLs). The curves represent the mean, and the error bars denote the standard errors of the results from the 13 participants who did not meet the clinical typhoid fever definition (NoTF), and the 7 who did (TF). The dashed lines represent the baseline values (day 0). Numbers in the “X” axis represent days after challenge, except for the numbers inside of the green box that represent 48 and 96 hrs after diagnosis of typhoid disease. Data are presented as absolute MAIT cell numbers per microliter of peripheral blood.