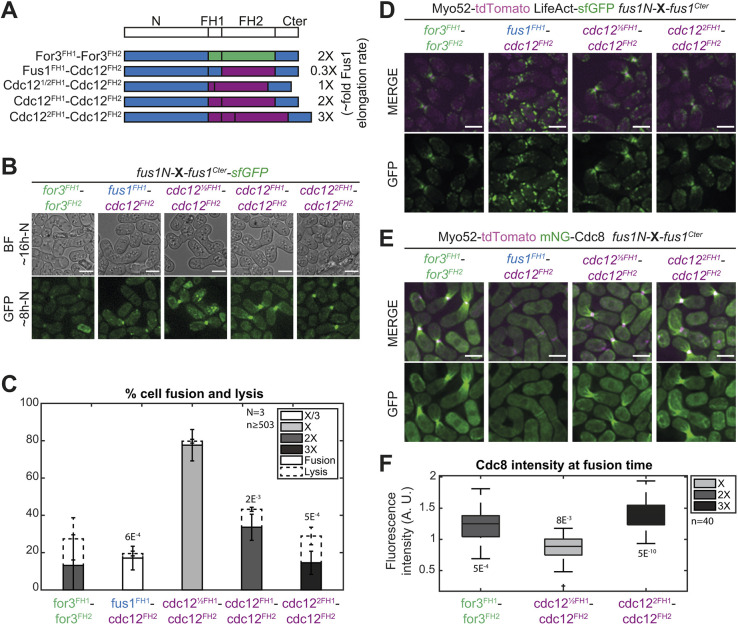
Fig. 5.
Low elongation and nucleation rates are detrimental for cell fusion and Fus1 contains an additional property within its FH2 domain absent from Cdc12 FH2 domain. (A) Scheme of the constructs used in the figure. All constructs were constructed seamlessly and integrated at the endogenous fus1 locus. As they keep their N- and C-terminal regulatory parts constant, they are referred to only by their variable FH1 and FH2 domains. Indicative actin filament elongation rates as measured in vitro on FH1-FH2 fragments by Scott et al. (2011) are shown on the right, as multiple of Fus1 elongation rate. Note that For3 exhibits ∼80-fold lower nucleation rates than Fus1 (not shown). (B) DIC images ∼16 h post starvation and GFP fluorescence images ∼8 h post starvation of homothallic strains expressing the formin chimeras shown in A, C-terminally tagged with sfGFP. (C) Percentage of cell pair fusion and lysis 24 h after nitrogen removal in strains as in B. P-values are relative to the most efficient chimera, Cdc12½FH1-Cdc12FH2. (D) Merge and GFP fluorescence images ∼8 h post starvation of Myo52-tdTomato and LifeAct-sfGFP in homothallic strains expressing untagged formin chimeras shown in A. (E) Merge and GFP fluorescence images ∼8 h post starvation of Myo52-tdTomato and mNG-Cdc8 in homothallic strains expressing untagged formin chimeras shown in A. (F) Boxplot of mNG-Cdc8 intensity normalized to the WT (shown in Fig. 4E) at the cell contact site at fusion time in strains as in E. P-values are relative to WT. Bars are 5 µm.