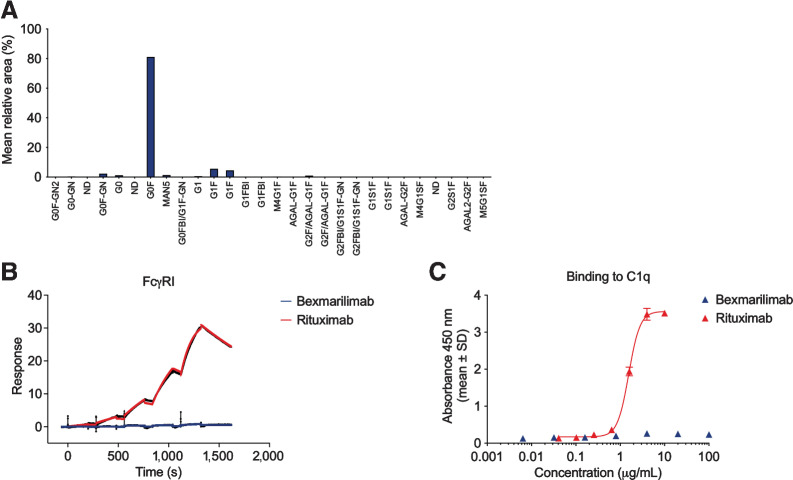
Figure 3.
Bexmarilimab does not contain immunogenic glycans or bind to human FcγRI or complement 1q. A, N-glycoform distribution on bexmarilimab as determined by quantitative N-glycan profiling. The analysis was performed in triplicate and glycans were assigned according to retention time. B, Binding of bexmarilimab and the positive control IgG rituximab to FcγRI as determined by SPR analysis. Colored lines correspond raw data, black lines correspond fitted data. C, Binding of bexmarilimab and rituximab to component C1q of the complement pathway as determined by ELISA; a representative graph of three experiments. s, seconds.