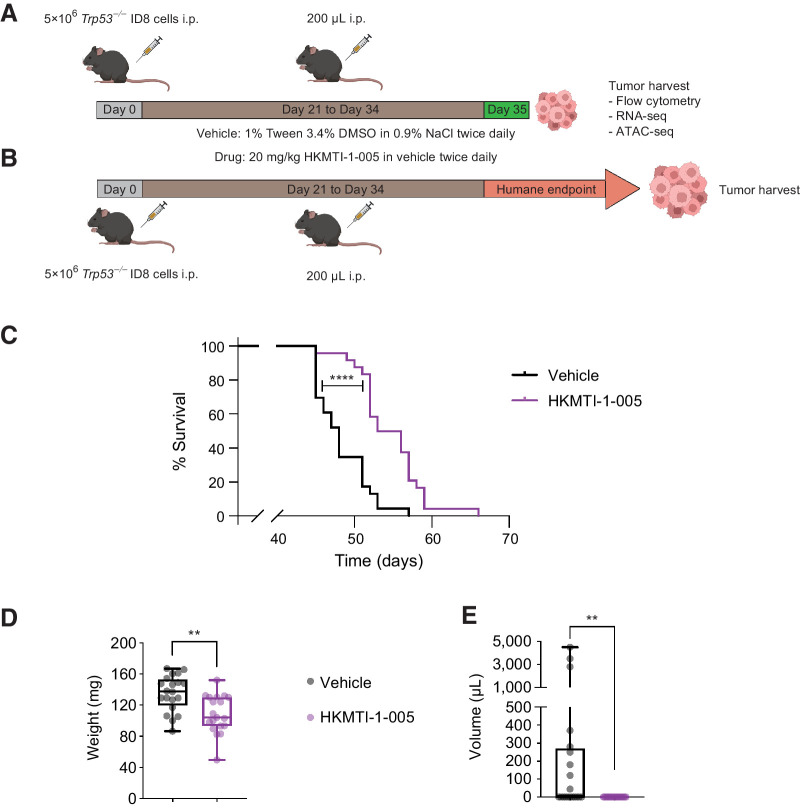
Figure 4.
Dual G9A/EZH2 inhibition inhibits tumor growth and prolongs survival in a mouse ovarian cancer model. A, Experimental design for mechanism experiments. Mice bearing intraperitoneal Trp53−/−ID8 cells were treated with either HKMTI-1–005 (20 mg/kg i.p. twice a day) or vehicle (1% Tween/3.4% DMSO in 0.9% NaCl i.p. twice a day) for 14 days starting on day 21, followed by omental and porta hepatic deposits harvest/weighting, measuring ascites and immunophenotyping by flow cytometry immediately after the end of treatment. Image created with BioRender.com. B, Experimental design for efficacy experiments. Mice were treated as per A, but treatment was followed by observation until mice reached humane survival endpoint. C, Kaplan–Meier survival curves for mice treated with vehicle (n = 24) or 20 mg/kg HKMTI-1–005 (n = 24) as per schedule on B. Median survival was 48 days for vehicle versus 54.5 days for HKMTI-1–005, P < 0.0001). Curves were compared using the Log-rank (Mantel–Cox) test, ****, P < 0.0001. Experiment was performed twice with n = 12 per cohort for each experiment. D, Whole tumor weight (including both porta hepatis and omental tumor deposits) and E ascites volume for mice treated with either vehicle (n = 20) or HKMTI-1–005 (n = 20) as per schedule in A; comparisons were made using unpaired t test for whole tumor burden and Mann–Whitney test for ascites volume (**, P < 0.01). Experiment was performed twice with n = 10 per cohort for each experiment.