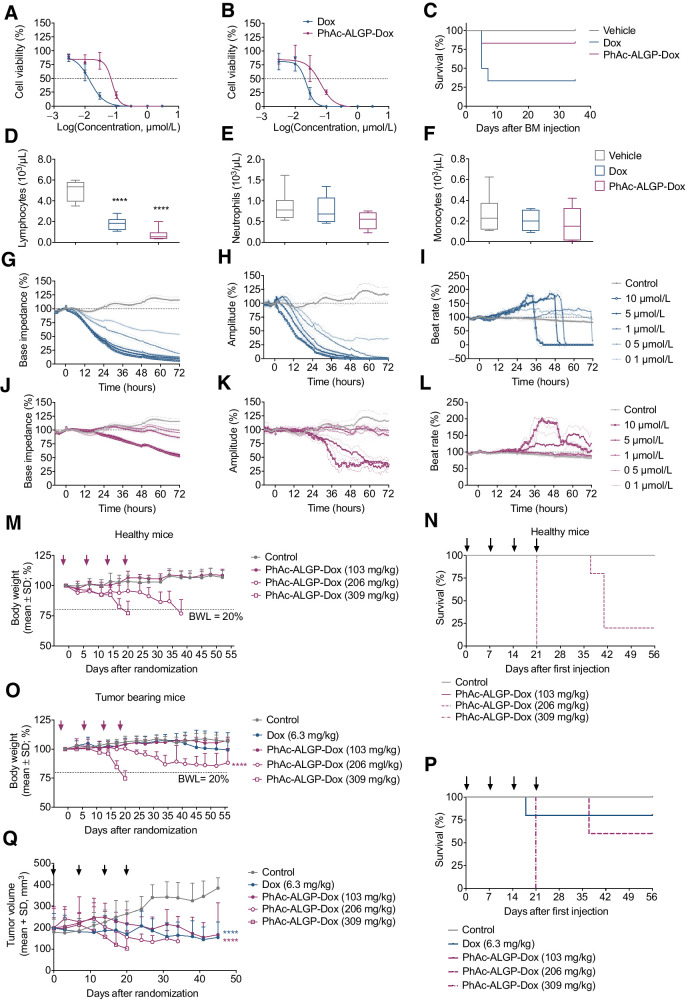
Figure 4.
PhAc-ALGP-Dox shows reduced hematotoxicity and cardiotoxicity as well as improved MTD with tumor sequestration in tumor-bearing mice. A and B, Dose–response curves for the effect of continuous exposure (14 days) to PhAc-ALGP-Dox on human erythroid (A) and myeloid (B) progenitor proliferation using MethoCult GF H84434. C, Kaplan–Meier plot describing the survival rate of recipient mice after BMT of vehicle, Dox (58 mg/kg/week) or PhAc-ALGP-Dox (1,026 mg/kg/week) treated donors. Mice were monitored for 41 days following BMT (n = 6). Gehan–Breslow–Wilcoxon test, P = 0.03. D–F, Blood count of donor mice treated with Dox (58 mg/kg/week) or PhAc-ALGP-Dox (1,026 mg/kg/week) by means of osmotic minipumps; whole blood was analyzed at the time of bone marrow collection. Data represent mean ± SD (n = 8). ****, P < 0.0001 versus control as defined by two-way ANOVA. G–L, Impedance-based cardiotoxicity of Dox (blue curves) and PhAc-ALGP-Dox (purple curves) in hiPSC-CMs on base impedance, contraction amplitude, and beat rate. Data of quadruplicate values are represented as mean ± SD. M–Q, PhAc-ALGP-Dox dose–response in healthy versus MDA-MB-468 tumor-bearing mice. Body weight (M, O), survival rate (N, P), and tumor volume (Q) were periodically monitored during and after the treatment (as indicated by arrows). Data represent mean ± SD (n = 5). ****, P < 0.0001 versus control (saline) as defined by two-way ANOVA; Gehan–Breslow–Wilcoxon test, P < 0.0001 (N) and P = 0.0013 (P). BMT, bone marrow transplantation; hiPSC-CM, human induced pluripotent stem cell derived cardiomyocyte.