Abstract
Background:
Orthostatic hypotension (OH) based on a change from seated-to-standing blood pressure (BP) is often used interchangeably with supine-to-standing BP.
Methods:
The Study to Understand Fall Reduction and Vitamin D in You (STURDY) was a randomized trial of vitamin D3 supplementation and fall in adults aged ≥70 years at high risk of falls. OH was defined as a drop in systolic or diastolic BP of at least 20 or 10 mmHg, measured at pre-randomization, 3-, 12-, and 24-month visits with each of 2 protocols: seated-to-standing and supine-to-standing. Participants were asked about orthostatic symptoms, and falls were ascertained via daily fall calendar, ad hoc reporting, and scheduled interviews.
Results:
Among 534 participants with 993 paired supine and seated assessments (mean age 76 ± 5 years, 42% women, 18% Black), mean baseline BP was 130 ± 19/68 ± 11 mmHg; 62% had a history of high BP or hypertension. Mean BP increased 3.5 (SE, 0.4)/2.6 (SE, 0.2) mmHg from sitting to standing, but decreased with supine to standing (mean change: –3.7 [SE, 0.5]/–0.8 [SE, 0.3] mmHg; P-value < 0.001). OH was detected in 2.1% (SE, 0.5) of seated versus 15.0% (SE, 1.4) of supine assessments (P < 0.001). While supine and seated OH were not associated with falls (HR: 1.55 [0.95, 2.52] vs 0.69 [0.30, 1.58]), supine systolic OH was associated with higher fall risk (HR: 1.77 [1.02, 3.05]). Supine OH was associated with self-reported fainting, blacking out, seeing spots and room spinning in the prior month (P-values < 0.03), while sitting OH was not associated with any symptoms (P-values ≥ 0.40).
Conclusion:
Supine OH was more frequent, associated with orthostatic symptoms, and potentially more predictive of falls than seated OH.
Keywords: blood pressure measurement, falls, light-headedness, orthostatic hypotension
INTRODUCTION
Orthostatic hypotension (OH) is a common condition among older adults that is associated with a higher risk of falls, dementia, and early death, as well as a higher burden of orthostatic symptoms.1–4 Guidelines on the measurement of OH are inconsistent - some recommend a supine-to-standing protocol and others describe a seated-to-standing protocol.5–7 In a recent meta-analysis, nearly all antihypertensive trials used a seated protocol as a safety parameter in the setting of more intensive hypertension treatment.8 However, the impacts of seated versus supine protocols on the detection of OH and their relationships with long-term events have not been adequately described.
The Study to Understand Fall Reduction and Vitamin D in You (STURDY) was a double-blind, randomized trial that tested the effects of four doses of vitamin D3 (200, 1000, 2000, and 4000 IU/day) on fall risk in older adults (70 years and older) with low serum 25-hydroxyvitamin D [25(OH)D] levels (10–29 ng/ml).9,10 STURDY did not find any benefits from high doses of vitamin D3 supplementation on fall risk or risk of seated OH.11 An ancillary study to STURDY implemented a supine OH protocol in addition to the seated protocol collected as part of the main trial.
The purpose of the present study was to compare (1) the prevalence of OH detected via a supine-to-standing versus a seated-to-standing assessment, (2) the association of supine versus seated OH with incident falls, and (3) the association of supine versus seated OH with orthostatic symptoms. We hypothesized that supine OH would be more prevalent, more predictive of falls, and more strongly associated with orthostatic symptoms than seated OH, which could inform how OH should be performed in clinical and research settings.
METHODS
STURDY was a National Institute on Aging-sponsored trial conducted between July 2015 and May 2019 in Maryland at two community-based research clinics (Hagerstown, MD; Woodlawn, MD). A description of the trial’s design and results are reported elsewhere.9,10 In July 2017, participants were invited to participate in a supine OH ancillary study funded by the National Heart, Lung, and Blood Institute. Both parent and ancillary studies were approved by the Johns Hopkins University Institutional Review Board. All participants provided informed consent in writing.
Participants
STURDY included community-dwelling, older adults, aged 70 years and older with low serum 25(OH)D levels between 10–29 ng/ml and elevated fall risk (i.e., 2 or more falls or 1 injurious fall in the past year, fear of falling, difficulty maintaining balance, or use of an assistive device). Adults with cognitive impairment, hypercalcemia, kidney stones, consuming >1000 IU/day of vitamin D3 supplements, or >1200 mg/day of calcium supplements were excluded.9,10
OH protocols
Seated OH was measured by trained staff as part of the STURDY trial at baseline, 3-, 12-, and 24-month visits. Participants sat for 5-min with their backs supported, legs uncrossed, and feet flat on the ground. Cuff size was chosen based on measured right arm circumference, and three seated blood pressure (BP) measurements were performed with an Omron HEM907XL (Omron Healthcare Inc., Lake Forest, IL, USA), separated by 30 s. Afterward, participants were asked to stand and rest their arm on a bedside table, positioned to support their arm at about the height of their heart at a 70–80 degree angle from their torso. After 1-min from the time both feet were on the ground, participants underwent 3 additional BP measurements separated by a 30-s pause prior to the initiation of the next measurement while standing.
The supine OH protocol was performed at the randomization visit (approximately 2 weeks after the baseline visit) and the 3-, 12-, and 24-month visits as part of an ancillary study, examining OH timing and position. At the 3-, 12-, and 24-month visits, supine OH was performed later on the same day immediately after the seated OH protocol. The protocol asked staff to perform the supine assessment prior to phlebotomy and physical function assessments. Regardless of the preceding activity, for the supine OH protocol, participants lied supine for 5 min. BP was then measured 3 times with a 5-s pause prior to the initiation of the next measurements with their arm lying by their side. Participants were then asked to stand up as quickly and safely as possible and place their arm on a pre-positioned bedside table where their arm could rest at heart level at about a 70–80 degree angle from their torso. Research staff members were permitted to provide assistance during the process of standing. If the participant felt dizzy or uncomfortable during standing, they could lean against the exam table, but otherwise were asked to stand away from the table. We performed 3 measurements immediately after both feet were on the ground (5-s pause between measurements) and another 3 measurements, timed at 3-min after standing (5-s pause between measurements).
Visits were performed both in the morning and afternoon of a typical workday. Participants were required to wait at least 30 min after smoking, exercise, meals, or caffeine prior to BP measurement.
Analyses were restricted to visits at which participants had paired seated and supine protocols, except for pre-randomization during which a participant had a seated measurement at the baseline visit and a supine measurement during the randomization visit. For both protocols, we determined the difference in mean standing and seated or supine BP, based on the average of all available measurements. There were up to 3 seated and up to 3 standing BP measurements for the seated protocol, and up to 3 supines and up to 6 standing BP measurements for the supine protocol. OH was based on the average of the up to 6 standing BP measurements and defined as a drop in systolic blood pressure (SBP) of at least 20 mmHg or a drop in diastolic BP of at least 10 mmHg, using thresholds described in the consensus definition.6 We also defined: (1) systolic OH as a drop in SBP of at least 20 mmHg regardless of the change in diastolic blood pressure (DBP), (2) diastolic OH as a drop in DBP of at least 10 mmHg regardless of the change in SBP, and (3) orthostatic hypertension as an increase in SBP of at least 20 mmHg or an increase in DBP of at least 10 mmHg.
Heart rate was measured at the time of all BP assessments. Orthostatic tachycardia was defined as an increase in heart rate after standing a least 20 beats per minute.
Falls
STURDY used the World Health Organization’s definition of a fall, which is, any fall, slip, or trip in which the participant lost balance and landed on the floor or ground or at a lower level.12 STURDY used three methods to ascertain falls over the up to two-year follow-up period: monthly calendars, scheduled clinic visits and telephone calls, and ad hoc telephone contacts (participants called the clinic if they fell).13 Participants were provided a calendar each month and were asked to document whether a fall occurred on their calendar daily. Incident and recurrent fall surveillance continued up to 2 years or until the study ended.
Orthostatic symptoms
As part of the supine OH protocol, but prior to the BP measurements, participants answered questions about the frequency of orthostatic symptoms experienced in the preceding 30-days, that is, the occurrence of light-headedness, dizziness, fainting, blacking out, seeing spots, imbalance, room spinning, racing heart, sweating episodes, vision changes, nausea, trouble concentrating, shortness of breath, headache, muscle weakness, and fatigue during the process of standing up. Participants answered using a 9-point Likert scale; symptoms were then dichotomized as none (score 1) or any symptoms (scores 2–9). These symptoms were assessed during the randomization visit and during the 3-, 12-, and 24-month visits.
Distinct from the symptoms above, after the seated OH protocol, participants were asked if they felt lightheaded or dizzy immediately after standing and again after the three standing BP measurements were completed. During the supine OH protocol, between the immediate and the 3-min delayed measurements, participants were asked the following on a 9-point Likert scale: “As you stood or as you are standing right now, on a scale from 1 to 9, with 1 being ‘no symptoms’ and 9 being the ‘worst possible’, please rate if you feel (or felt): dizziness, lightheadedness, faint, or like you might black out.”
Other covariates
Age, sex, race (non-Black, Black, or unknown), history of cardiovascular disease (yes or no), diabetes (yes or no), history of high BP or hypertension (yes or no), history of Parkinson’s disease (yes or no), fall in the past year (yes or no), and use of antidepressants, Parkinson’s disease medications, antipsychotic medications, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta blockers, calcium channel blockers, diuretics, central alpha agonists, and alpha blockers were self-reported. Body mass index (BMI, kg/m2) was calculated based on measured height and weight.
Analysis
We described the characteristics of the adults who had both a seated and supine OH assessment using means and proportions overall and according to seated or supine OH status, identified at any time during the study. In addition, we determined the proportion with OH according to protocol within age categories (70–74, 75–79, 80–84, and 85 years or older) and according to self-reported biologic sex. Changes in BP by protocol were visualized with scatter plots and Lowess curves.
We determined the prevalence of OH, systolic OH, and diastolic OH among all instances of OH, using generalized estimating equations (Poisson family, log link, robust variance estimator, exchangeable correlation matrix) to account for repeated OH measurements among participants. For differences in SBP or DBP after standing, we used generalized estimating equations with a normal family identity link (robust variance estimator, exchangeable correlation matrix). These models were repeated to examine heart rate and orthostatic tachycardia. We compared means and proportions between seated and supine protocols using generalized estimating equations as well. We also repeated these analyses restricted to post-randomization visits only, given the asynchrony between pre-randomization assessments.
The absolute risk of falls from randomization was visualized with cumulative incidence plots according to each OH protocol in our study’s analytic sample, restricted to participants with both seated and supine protocols as well as an unrestricted sample with unpaired supine or seated OH assessments. We determined the relative risks of the incident and subsequent falls associated with OH, systolic OH, diastolic OH, difference in SBP, and difference in DBP using Cox proportional hazards models following the Andersen-Gill approach for multiple failure survival analysis.14,15
Each OH metric was treated as a time-varying covariate. OH status was determined before fall events and updated at scheduled assessments (3-, 12-, 24-month visits). For both protocols, follow-up began with the randomization visit. All Cox models were adjusted for age, sex, race, and field center with a robust variance estimator. We also used restricted cubic splines to visualize the relationship between differences in SBP and DBP from seated or supine protocols with falls and compared the pseudo-likelihood ratios of models with and without the addition of supine or seated protocol splines, using likelihood ratio tests. In sensitivity analyses, we repeated these models: using first fall only, examining the supine OH adjusted for seated OH, examining supine OH based on a subset of three standing measurements for the average standing BP that most corresponded to the timing of the three seated OH protocol standing measurements (i.e., measurements 2–4 or measurements 3–5 of the 6 total), and adjusting for assigned vitamin D3 dose (200 IU/day vs 1000+ IU/day). We also examined orthostatic hypertension based on both supine and seated protocols.
In addition, we determined the relationship between OH from supine or seated protocols with orthostatic symptoms experienced in the preceding 30-days or identified during either protocol, using generalized estimating equations (binomial family, logit link, robust variance estimator, exchangeable correlation matrix).
RESULTS
Baseline characteristics
Of the 534 STURDY participants who underwent both seated and supine OH protocols, we identified 21 (3.9%) with seated OH and 109 (20.4%) with supine OH at any time during the study (Table 1). Of the 21 with seated OH, there were no OH recurrences. In contrast, there were 144 instances of supine OH observed among the 109 participants with supine OH. Compared to participants with OH identified via the supine protocol, participants with seated OH were more often women or Black adults, had a higher baseline seated BP, had a more frequent history of cardiovascular disease or hypertension, used more Parkinson medications, beta blockers or angiotensin-converting protocol and 424 without orthostatic hypotension with the supine protocol. enzyme inhibitors/angiotensin receptor blockers, and used fewer diuretics. Characteristics between those included in this ancillary and those excluded were comparable (Figure S1; Table S1).
TABLE 1.
Baseline characteristics, mean (SD) or %
| Overall N= 534 |
Seated |
Supine |
|||
|---|---|---|---|---|---|
| No OH, N= 513 | OH, N= 21 | No OH, N= 425 | OH, N= 109 | ||
| Age, year | 76.3 (5.3) | 76.2 (5.3) | 78.4 (6.3) | 76.2 (5.2) | 76.8 (5.7) |
| Men, % | 58.1 | 58.9 | 38.1 | 57.6 | 59.6 |
| Black, % | 18.0 | 17.3 | 33.3 | 17.4 | 20.2 |
| Baseline seated blood pressure, mmHg | |||||
| Systolic | 129.8 (18.6)a | 129.3 (18.5)a | 140.3 (19.9) | 129.6 (18.5)a | 130.3 (19.2) |
| Diastolic | 67.9 (10.7)a | 67.8 (10.8)a | 70.2 (7.8) | 68.0 (10.7)a | 67.5 (10.7) |
| Body mass index, kg/m2 | 30.2 (5.3) | 30.3 (5.3) | 29.4 (6.0) | 30.3 (5.3) | 29.8 (5.3) |
| History of cardiovascular disease, % | 28.8 | 28.5 | 38.1 | 28.7 | 29.4 |
| History of diabetes, % | 26.8 | 26.5 | 33.3 | 26.6 | 27.5 |
| History of Parkinson’s disease, % | 1.5 | 1.4 | 4.8 | 1.2 | 2.8 |
| History of high blood pressure or hypertension, % | 62.0 | 61.0 | 85.7 | 62.8 | 58.7 |
| Antidepressant use, % | 17.0 | 17.0 | 19.0 | 15.8 | 22.0 |
| Parkinson’s disease medication use, % | 0.6 | 0.7 | 0.0 | 0.0 | 0.0 |
| Antipsychotic use, % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| ACE inhibitor/ARB use, % | 47.4 | 46.4 | 71.4 | 48.7 | 42.2 |
| Beta blocker use, % | 29.6 | 29.0 | 42.9 | 30.6 | 25.7 |
| Calcium channel blocker use, % | 22.3 | 22.2 | 23.8 | 21.9 | 23.9 |
| Diuretic use, % | 19.7 | 20.1 | 9.5 | 20.2 | 17.4 |
| Central alpha agonist use, % | 0.7 | 0.8 | 0.0 | 0.9 | 0.0 |
| Alpha blocker use, % | 8.6 | 8.4 | 14.3 | 6.8 | 15.6 |
| Fall in the past year, % | 62.9 | 62.4 | 76.2 | 62.1 | 66.1 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; OH, orthostatic hypotension; SD, standard deviation.
There were only 533 with blood pressure measured in the seated position at baseline. This corresponds to 512 without orthostatic hypotension with the seated
Prevalence of OH and postural change in BP
The number with supine OH at any time during the study was greater than the number with seated OH at all ages and this difference was highest among adults age 85 years and older (Figure 1A,B). OH was higher among women compared to men when measured using the seated protocol, but lower among women compared to men when using a supine protocol. Of the 993 OH assessments (see Table S2), OH was identified in 2.1% (SE, 0.5) with a seated protocol and in 15.0% (SE, 1.4) with the supine protocol (Table 2); only 0.9% (SE, 0.3) had both seated and supine OH (Table S3). Furthermore, the mean difference in SBP/DBP (i.e., standing minus seated or supine) was 3.5 (SE, 0.4)/2.6 (SE, 0.2) mmHg with the seated protocol versus –3.7 (SE, 0.5)/–0.8 (SE, 0.3) with the supine protocol (Figure 2A,B). Mean BP in the seated position was lower than in supine or standing positions. Changes in BP elicited by either protocol followed a linear relationship (Figure S2). OH from the supine protocol was more common than the seated protocol across age categories and for both men and women (Table S4). Findings were similar in sensitivity analyses that excluded the pre-randomization visits (Table S5).
FIGURE 1.
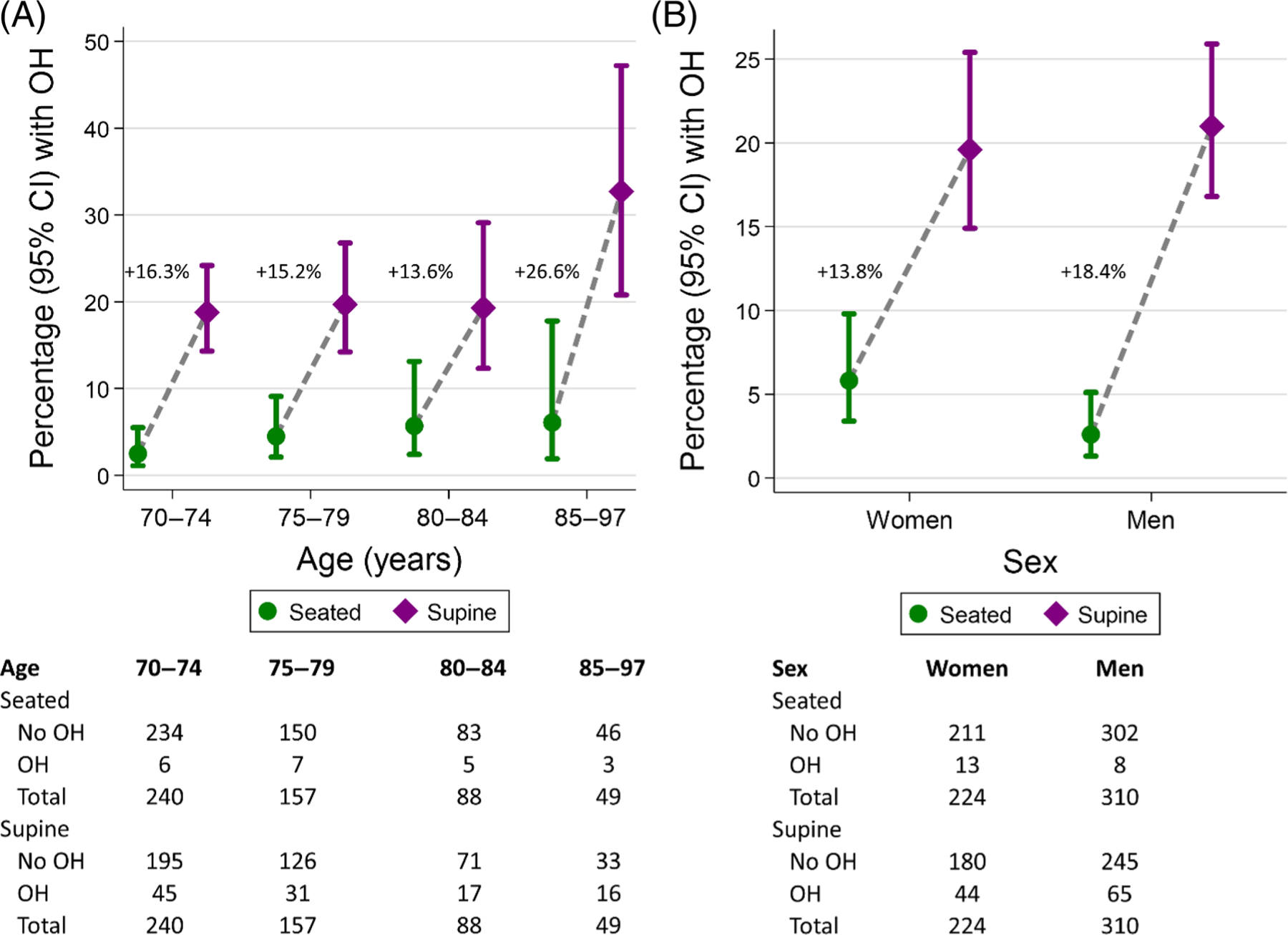
Unadjusted proportion (95% CI) with orthostatic hypotension at any time during the study according to seated (circle) or supine (diamond) protocols across (A) baseline age groupings: 70–74, 75–79, 80–84, and 85–97 years or greater or (B) self-reported biologic sex. Displayed is the absolute difference in proportion within each age or sex group
TABLE 2.
Prevalence of orthostatic hypotension and mean postural changes in blood pressure according to measurement protocol, N = 534 participants
| N of assessments | Seated protocol, % or mean (95% CI) | Supine protocol, % or mean (95% CI) | |
|---|---|---|---|
| OH, % | 993 | 2.1 (1.4, 3.2) | 15.0 (12.6, 18.0) |
| Systolic OH, % | 993 | 1.4 (0.8, 2.4) | 10.7 (8.6, 13.2) |
| Diastolic OH, % | 993 | 1.2 (0.7, 2.1) | 10.6 (8.6, 13.2) |
| Difference in SBP, mmHg | 993 | 3.5 (2.8,4.3) | −3.7 (−4.8,−2.6) |
| Difference in DBP, mmHg | 993 | 2.6 (2.2,3.0) | −0.8 ( −1.4,−0.3) |
Note: The seated protocol included up to 3 blood pressure measurements in the seated position after 5 min of rest followed by up to 3 measurements in the standing position 1 min after standing. All measurements were separated by 30 s. The supine protocol included up to 3 blood pressure measurements in the supine position after 5 min of rest followed by up to 3 measurements immediately after standing and at 3-min after standing (total 6 measurements). All measurements were separated by 5 s. Proportions were estimated using unadjusted generalized estimating equations.
Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; OH, orthostatic hypotension; SBP, systolic blood pressure.
FIGURE 2.
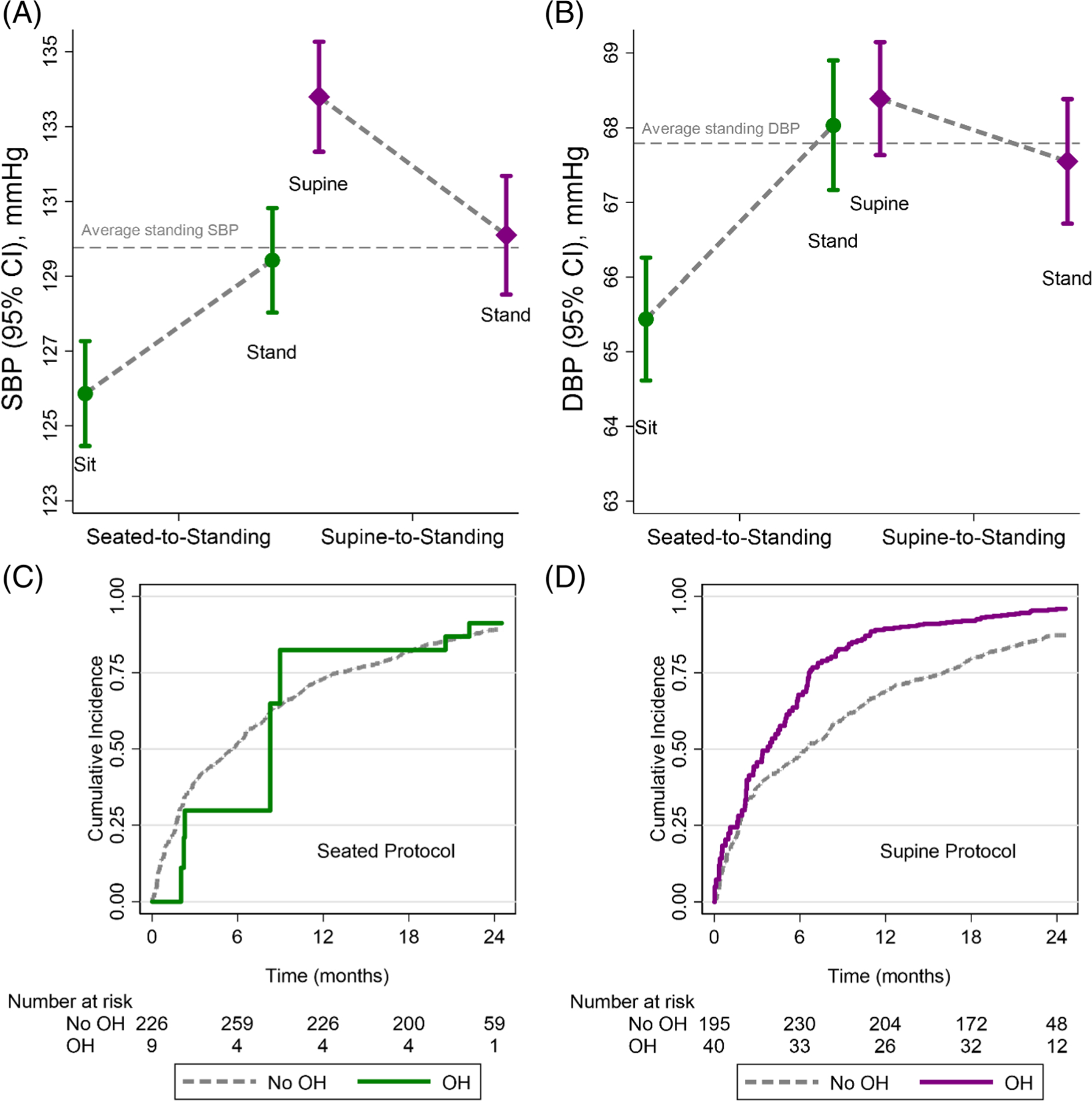
Mean (95% CI) (A) systolic blood pressure (SBP) and (B) diastolic blood pressure (DBP) in each position of the seated-tostanding or supine-to-standing protocols. The horizontal gray dashed line represents the mean standing blood pressure from both seated and supine protocols. Means were estimated using generalized estimating equations with a robust variance estimator. Cumulative incidence plots of the risk for recurrent fall events according to orthostatic hypotension status as a time-varying covariate identified using the (C) seated or (D) supine protocol. These plots are restricted to the population with both seated and supine protocols. Plots truncated at 750 days post-randomization
Examination of heart rate showed more frequent orthostatic tachycardia and greater increases in heart rate with the supine protocol (Figure S3; Table S6).
Association with falls
Among the 534 participants, who underwent the supine protocol at least once, 204 participants experienced at least 1 of the 481 falls observed over a median of 1.7 years of follow-up. The number of OH measures contributing to recurrent fall analyses is shown in Table S7. There was a stronger, yet non-significant, association between the risk of falls and OH from the supine protocol versus the seated protocol (Figure 2C,D) with a similar pattern using all available measurements (including non-concurrent OH assessments; Figure S4).
We observed non-linear relationships between change in BP and falls (Figure S5), and thus do not report associations with differences in SBP or DBP. While a supine SBP spline was significantly associated with falls (P = 0.0001), a seated SBP spline was not significantly associated with falls (P = 0.07). Splines of both seated DBP and supine DBP were significantly associated with falls (P = 0.0002 seated and P = 0.0006 supine).
While the hazard ratio of seated OH with falls was 0.69 (95% CI: 0.30, 1.58), the hazard ratio of supine OH with falls was 1.55 (95% CI: 0.95, 2.52) (Table 3). Supine systolic OH showed the greatest association with falls (HR: 1.77; 95% CI: 1.02, 3.05). Results were similar in sensitivity analyses (Tables S8–S13).
TABLE 3.
Association of orthostatic hypotension with falls, N = 534 participants
| Seated |
Supine |
|||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| OH | 0.69 (0.30, 1.58) | 0.38 | 1.55 (0.95, 2.52) | 0.08 |
| Systolic OH | 0.38 (0.10, 1.36) | 0.14 | 1.77 (1.02, 3.05) | 0.04 |
| Diastolic OH | 0.91 (0.38, 2.23) | 0.84 | 1.58 (0.88, 2.84) | 0.13 |
Note: Hazard ratios for time-to-fall were determined via Cox proportional hazards models. Falls were treated as recurrent events. Orthostatic hypotension was a time-varying covariate updated at pre-defined study time points (pre-randomization, 3-, 12-, and 24-month visits. Models were adjusted for age, sex, race, and field center. We ultimately did not display associations of difference in systolic and diastolic blood pressure with falls since these were not linear (see splines in Supporting information). Abbreviations: CI, confidence interval; HR, hazard ratio; OH, orthostatic hypotension.
Association with orthostatic symptoms
Seated OH was not statistically associated with any of the orthostatic symptoms examined during OH protocols or historic orthostatic symptoms in the previous 30 days (Tables S14 and S15). In contrast, supine OH was associated with significantly higher odds of fainting (OR: 3.46; 95% CI: 1.40, 8.55), blacking out (OR: 6.53; 95% CI: 2.01, 21.24), seeing spots (OR: 2.00; 95% CI: 1.25, 3.24), and room spinning (OR: 2.03; 95% CI: 1.09, 3.77).
DISCUSSION
In this population of older adults at higher risk of falling that underwent paired seated and supine OH protocols, a supine protocol identified substantially more OH than the seated protocol. The two protocols elicited distinct physiologic responses with the seated protocol being associated with a mean increase in BP, while the supine protocol was associated with a mean decrease in BP. Systolic OH from the supine protocol, but not from the seated protocol, was associated with falls. Moreover, OH from the supine protocol was associated with orthostatic symptoms in the preceding 30 days. These findings suggest that a supine protocol is more effective for detecting clinically relevant OH.
There is substantial variability in OH prevalence among older adults with reports of OH prevalence ranging from 2% to 28%.16–19 Much of this variability has been attributed to population characteristics. However, our study, like others,20–23 indicates that measurement protocol may be an important factor in the detection of OH. Our findings may help reconcile this heterogeneity in prevalence estimates in the literature, demonstrating stark differences in the prevalence of OH in the same population of older adults based on whether they were supine or seated prior to standing.
We found important differences in how BP changed according to the seated-to-standing and supine-to-standing protocols. We expected BP to drop to a greater extent with the supine protocol compared to the seated protocol. In contrast, BP rose upon standing from the seated protocol but was reduced upon standing from the supine position. We suspect these differences may reflect the effects of gravity on distinct blood volume distributions in rested supine and rested seated positions.28 However, the population is likely an important consideration here, as at least one other study of patients with predominantly neurogenic OH did not observe an increase in BP with seated-to-standing protocols.21
We found a non-significant trend that supine OH was more effective in identifying those at higher risk for falls than seated OH. Moreover, supine OH was associated with several orthostatic symptoms. This adds to the literature, demonstrating that beyond being more sensitive, supine OH better identify clinically relevant OH.
Our study has limitations. First, our sample size, while somewhat large, still may have been underpowered to detect associations with falls, and confidence intervals for the associations of OH with falls overlapped between seated and standing OH. Second, there were up to 6 assessments performed during the standing period of the supine protocol, which included the time immediately after standing, versus only 3 assessments for the seated protocol. Measures within 1-min have been found to better predict falls than later measures.1 While we can estimate result times during the seated measurement, exact times in this study were not recorded. However, sensitivity analyses where we restricted the supine assessment to the same number of measurements at a similar time interval did not meaningfully change the association of supine OH with falls. Third, supine protocol measurements were performed after seated protocol assessments. Thus, measures could be impacted by later times of the day or order effects often observed with BP measurement.24,25 Future studies that compare seated and supine protocols should randomize the order of assessments. Fourth, the pause between BP measures differed between seated and supine protocols. The shorter, 5-s pause may not be adequate for a washout of the effects of supra-systolic cuff inflation. Nevertheless, standing BPs were similar regardless of protocol. Fifth, we examined multiple orthostatic symptoms, increasing the possibility of falsely positive associations for individual symptoms. Thus, in our interpretation of these analyses, we focused on the pattern of associations rather than single symptoms. Sixth, our study included community-dwelling older adults at risk for falls. Neurogenic conditions were not assessed at baseline and were likely rare. This is an important distinction from a number of other studies on OH among institutionalized adults or patient populations recruited from specialty clinics (e.g., autonomic dysfunction).21,26 Seventh, while supine OH may be more clinically relevant, it is more difficult to standardize in research and clinical practice compared to a seated protocol.27 Eighth, symptoms regarding the prior 30 days were self-reported and may be subject to recall bias. Ninth, OH status changed between visits for a number of participants. However, there was not sufficient consistency in the number of visits to determine causes of change in OH status. This represents an important topic for subsequent research.
Our study has notable strengths. First, despite its observational design, all assessments were performed by highly trained staff in a standardized fashion and compared among the same participants, minimizing the effect of confounding characteristics. Second, our population was a diverse, community-based cohort of older adults at high risk of falls. While these findings might not generalize to populations with rarer forms of OH, for example, neurogenic OH,21 they are applicable among commonly encountered, ambulatory presentations among older adults in clinical practice. Third, STURDY used a highly robust fall ascertainment protocol, increasing our sensitivity to detecting falls. Moreover, we also examined a range of orthostatic symptoms, which are complementary to falls and represent potential pathologic contributors to fall events.
Our study has clinical implications. Both clinicians and researchers are frequently faced with the question of whether to assess OH using a supine or seated protocol, a decision often driven by time constraints. Many have viewed seated OH protocols as time saving or as a way to screen for persons that might need a more detailed supine assessment. Our results challenge this perspective, showing that the two procedures elicit distinct physiology and that the supine protocol may better identify adults at risk for orthostatic symptoms and perhaps falls. While research efforts to simplify and shorten assessments are needed, it is evident that a seated OH assessment cannot replace a supine OH assessment when monitoring for safety in response to a treatment or for determining subsequent fall risk.
Supplementary Material
Figure S1. Study population flow diagram.
Figure S2. Scatter plot of changes in blood pressure according to protocol.
Figure S3. Mean heart rate in each position of the seated-to-standing or supine-to-standing protocols.
Figure S4. Cumulative incidence plots of the risk for recurrent fall events in an unrestricted population that included noncurrent OH protocols.
Figure S5. Restricted cubic splines of change in blood pressure with falls.
Table S1. Baseline characteristics of those in the original trial were not included in this ancillary.
Table S2. Number of measurements contributing to each protocol.
Table S3. Cross-tabulation of orthostatic hypotension by protocol.
Table S4. Protocol and orthostatic hypotension according to age and biological sex.
Table S5. Protocol and orthostatic hypotension, excluding pre-randomization visits.
Table S6. Supine and seated protocols and orthostatic heart rate.
Table S7. Tabulation of the number of orthostatic hypotension assessments by a maximum number of observed falls.
Table S8. Association of orthostatic hypotension with first fall only.
Table S9. Association of supine protocol orthostatic hypotension with falls adjusted for orthostatic hypotension from the seated protocol.
Table S10. Association of supine protocol orthostatic hypotension with falls whereby the supine orthostatic hypotension was based on a subset of 3 measurements.
Table S11. Association of orthostatic hypotension with falls adjusted for randomization assignment.
Table S12. Association of orthostatic hypotension and orthostatic hypertension with falls.
Table S13. Association of orthostatic hypotension based on both seated and supine protocols with falls.
Table S14. Association of orthostatic hypotension with orthostatic symptoms in the preceding 30 days.
Table S15. Association of orthostatic hypotension with functional symptoms in the preceding 30 days or orthostatic symptoms during the orthostatic hypotension protocol.
Key points.
In this community-based population of older adults at risk for falls, seated and supine protocols elicited distinct physiologic responses, suggesting that are not interchangeable.
Orthostatic hypotension detected via a supine protocol was more common and more strongly associated with orthostatic symptoms and perhaps falls than orthostatic hypotension from a seated protocol.
Why does this paper matter?
In clinical practice, a seated protocol should not be considered a substitute for a supine protocol to screen for orthostatic hypotension.
ACKNOWLEDGMENTS
We thank participants of the STURDY study who volunteered in support of this research. STURDY is registered on clinicaltrials.gov under identifier NCT02166333.
SPONSOR’S ROLE
The funding agency had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Funding information
SPJ is supported by NIH/NHLBI 7K23HL135273. KJM is supported by NIH/NIA 5K24AG065525. STURDY was funded by the National Institute on Aging (U01AG047837) with support from the Office of Dietary Supplements, the Mid-Atlantic Nutrition Obesity Research Center (P30DK072488), and the Johns Hopkins Institute for Clinical and Translation Research (UL1TR003098). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med 2017;177:1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juraschek SP, Daya N, Appel LJ, et al. Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens 2017;30:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawlings AM, Juraschek SP, Heiss G, et al. Association of orthostatic hypotension with incident dementia, stroke, and cognitive decline. Neurology 2018;91:e759–e768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juraschek SP, Miller ER 3rd, Appel LJ. Orthostatic hypotension and symptoms in the AASK trial. Am J Hypertens 2018;31:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017;71: 1269–1324. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res 1996;6:125–126. [DOI] [PubMed] [Google Scholar]
- 7.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69–72. [DOI] [PubMed] [Google Scholar]
- 8.Juraschek SP, Hu J-R, Cluett JL, et al. Effects of intensive blood pressure treatment on orthostatic hypotension: a systematic review and individual participant-based meta-analysis. Ann Intern Med 2021;174:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michos ED, Mitchell CM, Miller ER 3rd, et al. Rationale and design of the Study To Understand Fall Reduction and Vitamin D in You (STURDY): a randomized clinical trial of Vitamin D supplement doses for the prevention of falls in older adults. Contemp Clin Trials 2018;73:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appel LJ, Michos ED, Mitchell CM, et al. The effects of four doses of vitamin D supplements on falls in older adults: a response-adaptive, randomized clinical trial. Ann Intern Med 2021;174:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juraschek SP, Miller Rd ER, Wanigatunga AA, et al. Effects of vitamin D supplementation on orthostatic hypotension: results from the STURDY trial. Am J Hypertens 2022. Feb 1;35(2):192–199. doi: 10.1093/ajh/hpab147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO Global Report on Falls Prevention in Older Age World Health Organization; 2008. [Google Scholar]
- 13.Teister CJ, Chocano-Bedoya PO, Orav EJ, et al. Which method of fall ascertainment captures the most falls in prefrail and frail seniors? Am J Epidemiol 2018;187:2243–2251. [DOI] [PubMed] [Google Scholar]
- 14.FAQ. Analysis of multiple failure-time survival data. Stata [Internet] September 9, 2021. https://www.stata.com/-support/faqs/statistics/multiple-failure-time-data/ [Google Scholar]
- 15.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat 1982;10:1100–1120. [Google Scholar]
- 16.Shin C, Abbott RD, Lee H, Kim J, Kimm K. Prevalence and correlates of orthostatic hypotension in middle-aged men and women in Korea: the Korean Health and Genome Study. J Hum Hypertens 2004;18:717–723. [DOI] [PubMed] [Google Scholar]
- 17.Strogatz DS, Keenan NL, Barnett EM, Wagner EH. Correlates of postural hypotension in a community sample of elderly blacks and whites. J Am Geriatr Soc 1991;39:562–566. [DOI] [PubMed] [Google Scholar]
- 18.Räihä I, Luutonen S, Piha J, Seppänen A, Toikka T, Sourander L. Prevalence, predisposing factors, and prognostic importance of postural hypotension. Arch Intern Med 1995;155:930–935. [DOI] [PubMed] [Google Scholar]
- 19.Saedon NI, Tan MP, Frith J. The prevalence of orthostatic hypotension: A systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2020. Jan 1;75(1):1177–122. doi: 10.1093/gerona/gly188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen S, He T, Chu J, He J, Chen X. Uncontrolled hypertension and orthostatic hypotension in relation to standing balance in elderly hypertensive patients. Clin Interv Aging 2015;10: 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw BH, Garland EM, Black BK, et al. Optimal diagnostic thresholds for diagnosis of orthostatic hypotension with a ‘sit-to-stand test’. J Hypertens 2017;35:1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooke J, Carew S, O’Connor M, Costelloe A, Sheehy T, Lyons D. Sitting and standing blood pressure measurements are not accurate for the diagnosis of orthostatic hypotension. QJM 2009;102:335–339. [DOI] [PubMed] [Google Scholar]
- 23.Braam EAJE, Verbakel D, Adiyaman A, Thien T. Orthostatic hypotension: revision of the definition is needed. J Hypertens 2009;27:2119–2120; author reply 2120. [DOI] [PubMed] [Google Scholar]
- 24.Kawano Y. Diurnal blood pressure variation and related behavioral factors. Hypertens Res 2011;34:281–285. [DOI] [PubMed] [Google Scholar]
- 25.van der Hoeven NV, Lodestijn S, Nanninga S, van Montfrans GA, van den Born B-JH. Simultaneous compared with sequential blood pressure measurement results in smaller inter-arm blood pressure differences. J Clin Hypertens (Greenwich) 2013;15:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman R, Illigens BMW, Lapusca R, et al. Symptom recognition is impaired in patients with orthostatic hypotension. Hypertension 2020;75:1325–1332. [DOI] [PubMed] [Google Scholar]
- 27.van Twist DJL, Harms MPM, van Wijnen VK, et al. Diagnostic criteria for initial orthostatic hypotension: a narrative review. Clin Auton Res 2021;31:685–698. [DOI] [PubMed] [Google Scholar]
- 28.Rowell LB. Human Cardiovascular Control Oxford University Press; 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study population flow diagram.
Figure S2. Scatter plot of changes in blood pressure according to protocol.
Figure S3. Mean heart rate in each position of the seated-to-standing or supine-to-standing protocols.
Figure S4. Cumulative incidence plots of the risk for recurrent fall events in an unrestricted population that included noncurrent OH protocols.
Figure S5. Restricted cubic splines of change in blood pressure with falls.
Table S1. Baseline characteristics of those in the original trial were not included in this ancillary.
Table S2. Number of measurements contributing to each protocol.
Table S3. Cross-tabulation of orthostatic hypotension by protocol.
Table S4. Protocol and orthostatic hypotension according to age and biological sex.
Table S5. Protocol and orthostatic hypotension, excluding pre-randomization visits.
Table S6. Supine and seated protocols and orthostatic heart rate.
Table S7. Tabulation of the number of orthostatic hypotension assessments by a maximum number of observed falls.
Table S8. Association of orthostatic hypotension with first fall only.
Table S9. Association of supine protocol orthostatic hypotension with falls adjusted for orthostatic hypotension from the seated protocol.
Table S10. Association of supine protocol orthostatic hypotension with falls whereby the supine orthostatic hypotension was based on a subset of 3 measurements.
Table S11. Association of orthostatic hypotension with falls adjusted for randomization assignment.
Table S12. Association of orthostatic hypotension and orthostatic hypertension with falls.
Table S13. Association of orthostatic hypotension based on both seated and supine protocols with falls.
Table S14. Association of orthostatic hypotension with orthostatic symptoms in the preceding 30 days.
Table S15. Association of orthostatic hypotension with functional symptoms in the preceding 30 days or orthostatic symptoms during the orthostatic hypotension protocol.


