Figure 1. TLRs signaling in the brain.
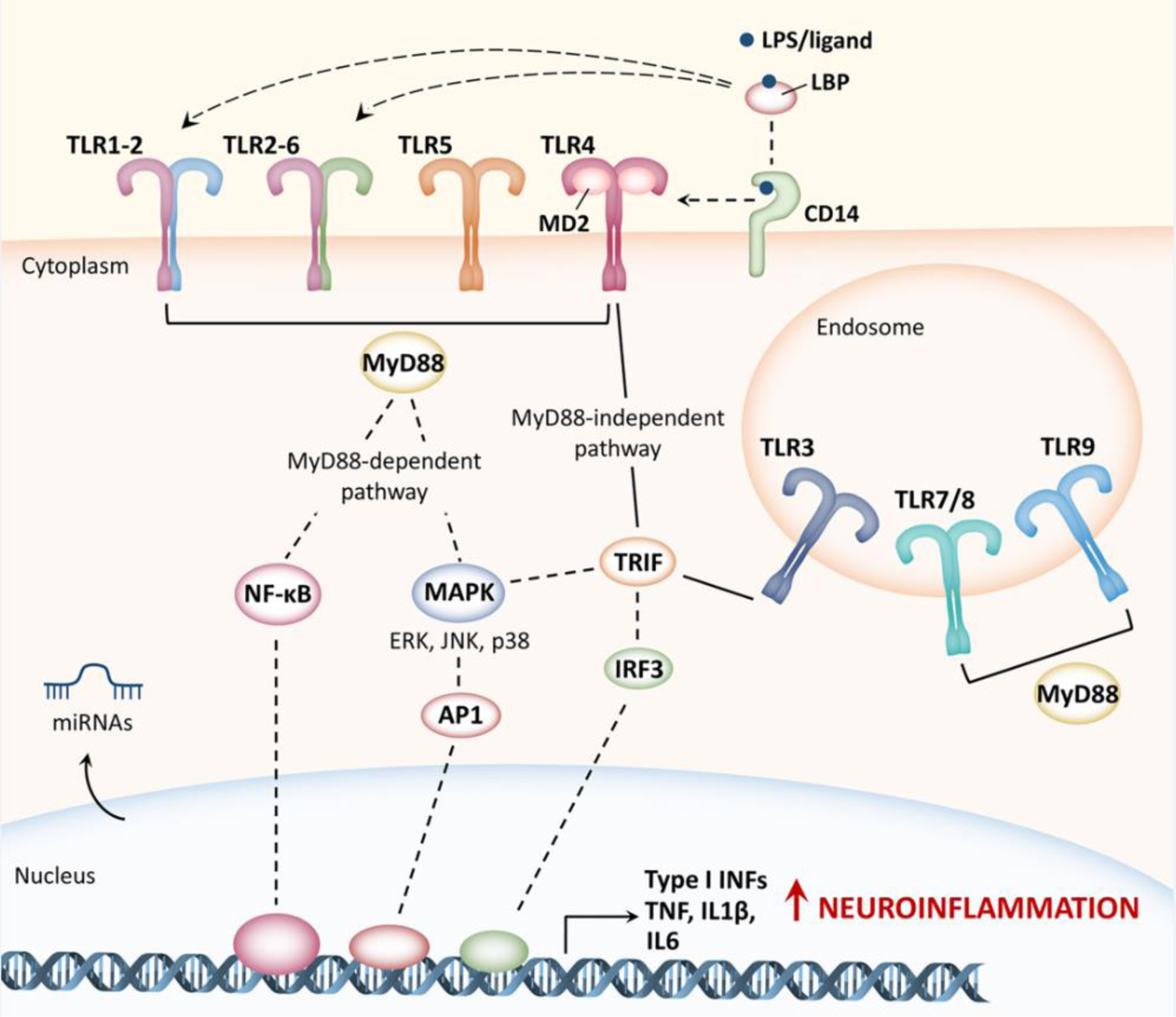
TLRs are expressed on the cell surface as well as in endosomes, and mainly in immune and glial cells. TLRs activation is extremely complex and requires multiple accessory proteins (including cluster differentiation-14, CD14, lipopolysaccharide-binding protein, LBP, and myeloid differentiation factor 2, MD2) that participate in ligand recognition and TLRs conformational changes. Following stimulation, TLRs form both homo- and heterodimers that triggers two main signaling cascades, depending on the adaptor protein involved: the myeloid differentiation primary response protein 88 (MyD88)-dependent pathway) or the TIR domain-containing adaptor-inducing interferon-β (TRIF) MyD88-independent pathway). Downstream signaling networks include the activation of different transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), mitogen-activated protein kinase (MAPK) and interferon regulatory factors (IRFs, including IRF3) ultimately leading to the production of inflammatory cytokines (TNF, IL1β, IL6) including Type I interferons (Type I INFs), which are responsible for T lymphocyte stimulation and recruitment. Noteworthy, TLR-mediated inflammatory cascades (in particular TLR2 and TLR4) in both neurons and glia overlap in normal aging and in the onset of different forms of dementia. TLRs activation can also drive the transcription of micro RNAs (miRNAs), which, in turn, can either stimulate or block TLRs activation. This is particularly interesting as multiple miRNAs are implicated in the pathogenesis of different diseases associated with cognitive impairment. ERK, extracellular signal-regulated kinases; JNK, c-Jun N-terminal kinases; AP1, activator protein 1.
