Scheme 1. Synthesis of compounds 1–11a.
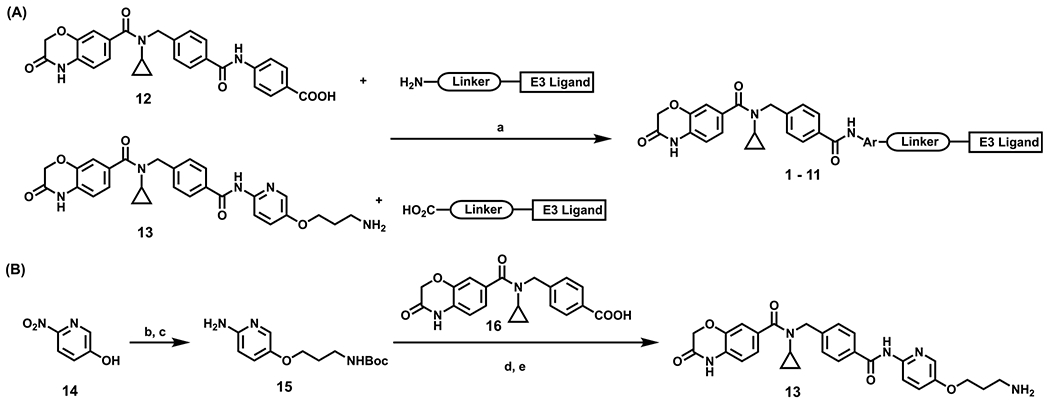
aReaction conditions: (a) HOAT, NMM, EDCI, DMSO, corresponding linker–E3 ligand, rt; (b) Cs2CO3, DMF, tert-butyl (3-bromopropyl)carbamate, 80 °C; (c) Pd/C, H2, MeOH, rt; (d) 16, SOCl2, 40 °C, remove solvent, then 15, pyridine, DMAP, rt; (e) DCM, TFA, rt.
