Figure 6. hRap1 modulates the phase-separation propensity of TRF2 with ds and ss nucleic acids.
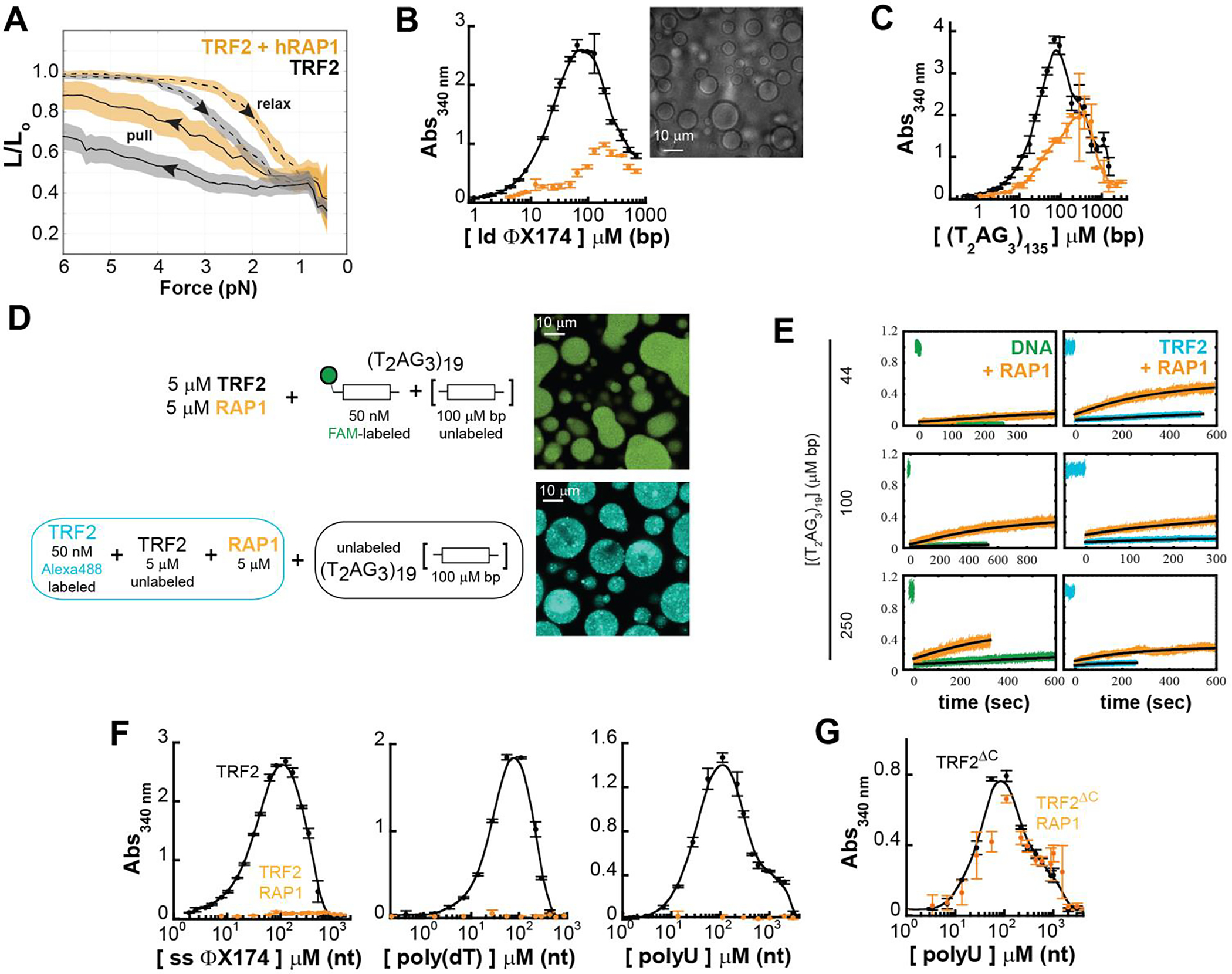
A. Force-extension experiments reveal a smaller TRF2-dependent DNA compaction in presence of stoichiometric concentration of hRap1 (dashed and solid lines are the average and color bands the standard deviation of multiple traces in either the relax or pull direction, respectively). B-C. Turbidity measurements of TRF2 and a TRF2- hRap1 stoichiometric complex in presence of non-specific (ϕX174 HaeIII digest) and specific (T2AG3)135 dsDNA. Addition of hRap1 does not ablate phase separation but modulates the phase-boundaries. D-E Phase separation of TRF2 (5 μM mon) and hRap1 (5 μM) with (T2AG3)19. Alexa Fluor 488-labeled TRF2 and FAM-labeled DNA demonstrate partitioning of the protein and DNA in the dense phase. FRAP measurements reveal that addition of hRap1 increases under all conditions the recovery of both DNA and TRF2. F. Turbidity measurements of TRF2 in presence of ss ϕX174, poly(dT), and poly(U) reveal suppression of phase separation upon addition of stoichiometric concentrations of hRap1. G. Turbidity measurements of the truncation variant TRF2ΔC, which lacks the binding site of hRap1. No change in turbidity is observed upon addition of hRap1.
