Figure 1. IsdB immunization is not protective in mice previously infected with S. aureus.
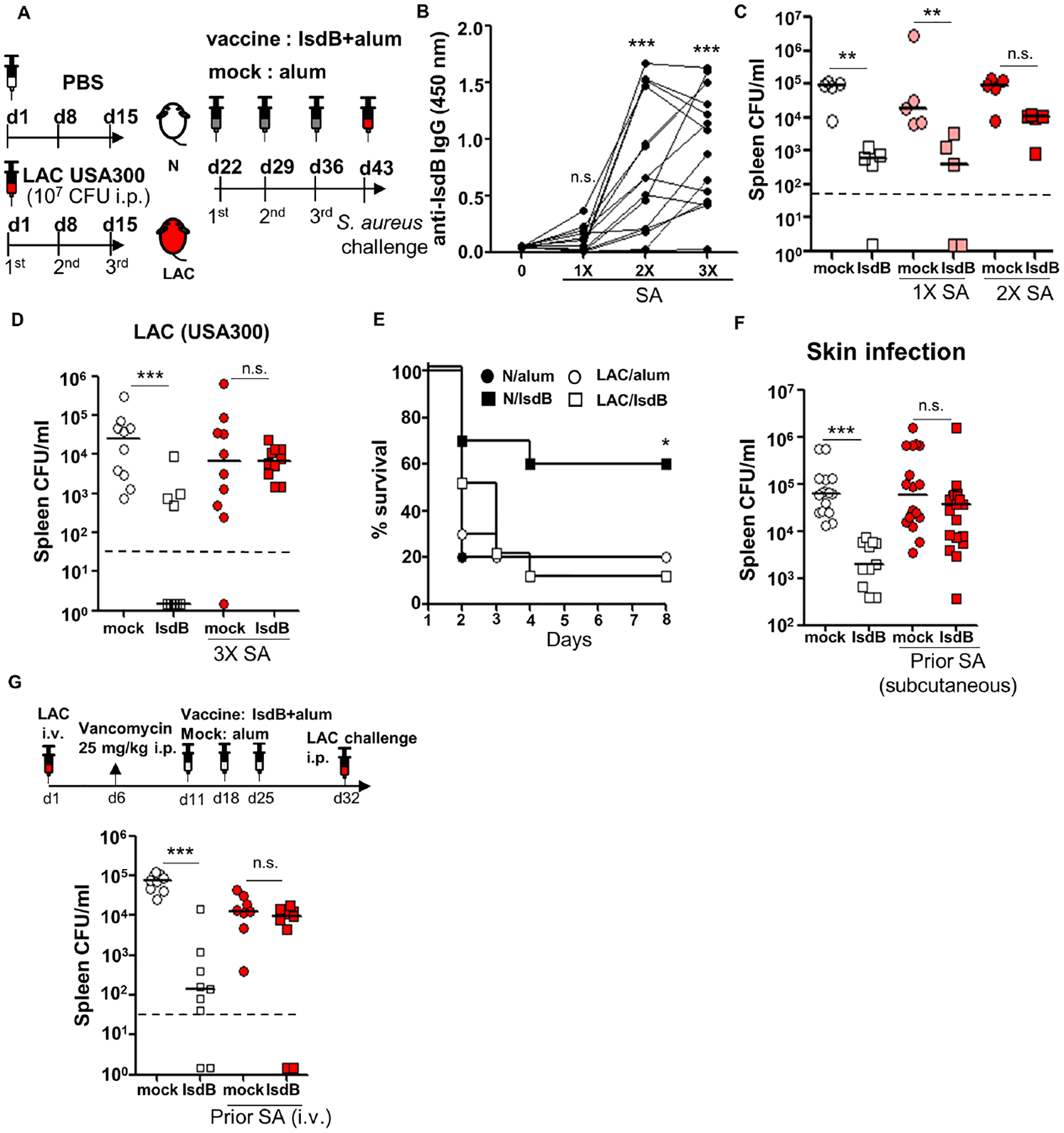
(A) Experimental setting. C57BL/6 mice injected intraperitoneally (i.p.) with 2×107 SA (LAC) or phosphate-buffered saline (PBS) 3 times at 7d intervals, immunized i.p. with IsdB plus alum (IsdB) or alum alone (Mock), then challenged with 2×107 LAC i.p.
(B) Serum anti-IsdB IgG after 1–3 LAC infections (n=15 per condition).
(C and D) Tissue bacterial burden in mice infected 1–3 times with LAC, then immunized and LAC challenged as per Figure 1A. Bacterial burden was measured 24 hrs after the last infection. N/IsdB: Naïve mice vaccinated with IsdB, N/alum: naïve mice given adjuvant alone; LAC/IsdB: LAC-infected mice vaccinated with IsdB; LAC/alum: LAC-infected mice given adjuvant alone (LAC/alum). N=5 for (C) and n=10 for (D) per mouse group.
(E) Kaplan-Meyer plot of mice treated as in Figure 1A with a final LAC challenge dose of 5×107 (LD90) (n=10 per mouse group).
(F) Tissue bacterial burden in mice infected subcutaneously (twice 2 weeks apart) with SA (LAC), IsdB immunized two weeks later and then LAC challenged i.p. per Figure 1A (n=11–19 per mouse group).
(G) Tissue bacterial burden in mice infected once i.v. with SA, treated for 5 days with vancomycin and then immunized and LAC challenged i.p. per Figure 1A (n=9 per mouse group).
Each data point represents an individual mouse; bars denote median and dashed lines indicate the limit of detection (C, D, F and G). n.s., not significant, *P<0.05, **P<0.01, and ***P< 0.001; one-way ANOVA (B to D, F and G) or long rank test (E). See also Figure S1.
