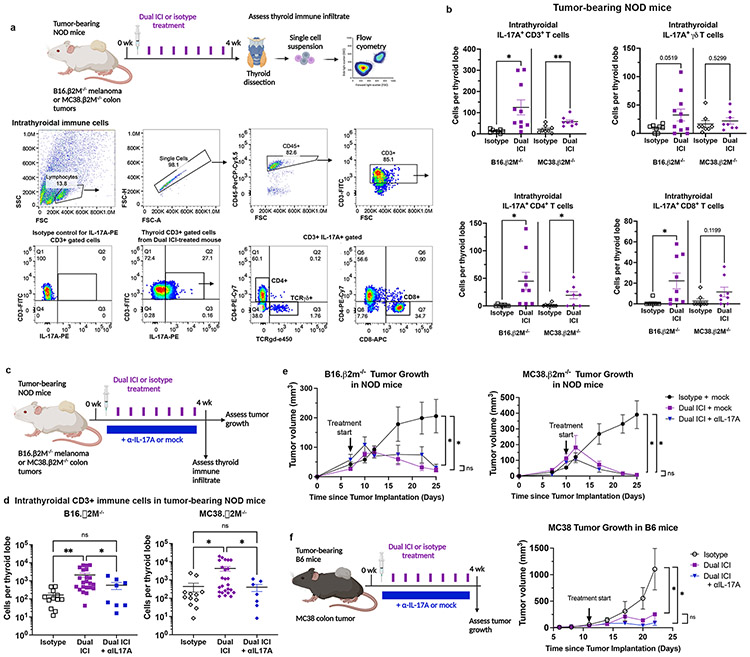
Figure 5. Contribution of IL-17A+ T cells in ICI-associated thyroid autoimmune infiltrates in tumor-bearing NOD mice.
a, Schematic of ICI treatment and thyroid immune infiltrate assessment in tumor-bearing NOD mice (top). Representative dot plots and gating strategy for thyroid infiltrating immune cells (bottom). b, Quantification of intrathyroidal IL-17A+ CD3+ T cells and IL-17A+ T cell subsets in tumor-bearing NOD mice by flow cytometry. Data shown as absolute cells per thyroid lobe; each dot represents one animal; stratified by tumor type. c, Schematic of ICI treatment with or without a neutralizing IL-17A (αIL-17A) antibody in tumor-bearing NOD mice for concurrent assessment on thyroid autoimmune infiltrate (IrAE) and tumor growth (ICI efficacy). d, Comparison of accumulation of intrathyroidal CD3+ T cells in isotype, Dual ICI, or Dual ICI + αIL-17A by flow cytometry. Data shown as absolute cells per thyroid lobe; each dot represents one animal; stratified by tumor type. e, Growth of B16 β2M−/− and MC38. β2M−/− tumors in NOD mice treated with isotype for ICI (isotype) and isotype for αIL-17A (mock) (n=11-13), Dual ICI with mock (n=7), or Dual ICI with αIL-17A (n=8). f, Growth of syngeneic MC38 colon tumors in C57/B6 (B6) mice treated with isotype, Dual ICI, or Dual ICI and αIL17A, n=6-8 each group. Data are mean ±SEM shown. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Two-tailed, unpaired t test with Welch correction, assuming unequal s.d. and Holm-Sidak method correction for multiple comparisons (b); Brown-Forsythe ANOVA, assuming unequal s.d., followed by Dunnett’s multiple comparisons test (d; comparison of day 25 tumor volume in e; comparison of day 22 tumor volume in f).