Abstract
Background: Odontogenic infections, and especially endodontic infections, are polymicrobial, involving a combination of Gram-positive and Gram-negative facultative anaerobes and strictly anaerobic bacteria. Therefore, antibiotics can be used as an adjunct to endodontic treatment. However, most chronic and even acute endodontic infections can be successfully managed by disinfection of the root-canal system, which eliminates the source of infection, followed by abscess drainage or tooth extraction, without the need for antibiotics. The literature provides evidence of inadequate prescribing practices by dentists. The aim of this concise review was to analyse the worldwide pattern of antibiotic prescription in endodontic infections. Methods: Comprehensive searches were conducted in MEDLINE/PubMed, Wiley Online Database, Web of Science and Scopus. The databases were searched up to 13 March 2016 for studies in which dentists used systemic antibiotics to treat endodontic lesions and which reported data on the type of antibiotic prescribed and on the diagnosis of the endodontic disease treated. Results: The electronic and hand searches identified 69 titles, of which 25 were included in the final analysis. Amoxicillin was reported as the drug of choice for endodontic infections in most countries, and clindamycin and erythromycin were the choice for patients allergic to penicillin. Dentists worldwide prescribe antibiotics for non-indicated conditions, such as pulpitis. Conclusion: Antibiotics are overprescribed for the management of endodontic infections. It is necessary to improve antibiotic-prescribing habits in the treatment of endodontic infections, as well as to introduce educational initiatives to encourage the coherent and proper use of antibiotics in such conditions.
Key words: Antibiotics prescription, pulpitis, apical periodontitis
Introduction
Alexander Fleming discovered the first antibiotic, penicillin, in 1928. Florey, in 1940, introduced the use of antibiotics to clinical practice. Since then, dentists have used antibiotics widely. However, whereas many bacteria were initially found to be sensitive to different types of antibiotics, there has been a continuing appearance of antibiotic-resistant strains1. Antibiotic resistance is tolerance of a microorganism to an antibiotic that was initially effective for treatment of infections caused by that microorganism. It has been noticed that some bacteria, including those implicated in apical periodontitis2, are developing resistance to most antibiotics currently available. Taking into account that dentists prescribe approximately 10% of all antibiotics commonly used, the impact of dentists in antimicrobial resistance is considerable3. After analgesics, antibiotics are the drugs most commonly prescribed by dentists4. A survey carried out in the UK in 2004 revealed that 40% of general dental practitioners prescribed antibiotics three times per week, and 15% prescribed them on a daily basis1. Nevertheless, it has been documented that such prescribing habits are either inappropriate or superfluous. Recently, it has been highlighted that the literature provides evidence of erroneous prescribing practices by dentists for a number of reasons, ranging from inadequate knowledge to social factors5.
Odontogenic infections, and especially endodontic infections, are polymicrobial, involving a combination of Gram-positive and Gram-negative facultative anaerobes and strictly anaerobic bacteria6., 7.. Therefore, systemic antibiotics can be used as an adjunct to endodontic clinical treatment whenever the host response cannot contain the infection8, such as in cases of persistent or systemic infections and in immunocompromised patients. The prescription of antibiotics influenced by patient demand or by the expectation of referring dentists is inappropriate9.
The aim of this review was to analyse the worldwide pattern of prescription of antibiotics by dentists for endodontic infections.
Materials and Methods
The question addressed in this review was: What is the worldwide pattern of antibiotics prescription by dentists in endodontic infections? The search strategy was as follows. Searches of MEDLINE/PubMed, Wiley Online Database, Web of Science and Scopus were performed using the following combination of Mesh terms and key words: (antibiotic OR antibacterial agents) AND (dentist OR endodontist) AND (prescription OR inappropriate prescribing OR prescription drug misuse OR drug overuse OR prescription drug overuse) AND (dental pulp diseases OR pulpitis OR dental pulp necrosis OR periapical diseases OR periapical periodontitis OR periapical abscess OR apical periodontitis). The bibliography of all relevant papers was hand-searched.
Three investigators (J.M-G., M.C.J-S. and J.J.S-E.) screened the titles and abstracts of all articles identified in the electronic and manual searches, according to the following inclusion criteria: (i) studies published from 1 January 1996 to 13 March 2016 (there were no restrictions according to sample age, specific features or study design); and (ii) articles reporting data on the type of antibiotic prescribed and the diagnosis of the treated endodontic disease. Articles that did not meet the inclusion criteria were excluded. All remaining articles were obtained and the full text was reviewed independently by four reviewers (E.V-O., I.C-G., J.J.S-M. and J.J.S-E), who included the studies investigating the antibiotic-prescribing patterns in the management of pulpitis and apical periodontitis. In the event of disagreement between authors, articles were discussed until consensus was reached. Data were extracted (E.V-O. and J.J.S-E.), synthesised and analysed. For each study, the following parameters were recorded: prescriber; country; diagnosis of endodontic diseases treated; first- and second-choice antibiotic; antibiotic choice in penicillin-allergic patients; duration of treatment; year of publication; and evidence level, determined according to guidelines provided by The Centre for Evidence-Based Medicine at Oxford10.
Results
The electronic and hand searches identified 69 titles (Figure 1). Duplicate references (20 items) and articles published before 1996 (four items) were discarded. A subsequent search through the titles and abstracts of the remaining 45 records revealed 31 articles for full-text reading. At this level, six studies were excluded because they did not provide data on the diagnosis of endodontic diseases treated11., 12., 13., 14., 15., 16.. In the final analysis, 25 studies were included. Table 1 shows studies according to continent, and summarises prescribers, diagnosis of endodontic diseases, first- and second-choice antibiotics, antibiotic choice in penicillin-allergic patients, treatment duration, publication year and study evidence level10.
Figure 1.
Flow chart showing the selection process for the studies included in the review.
Table 1.
Pattern of antibiotic prescription by dentists in different regions and countries
| Region and Country | P | IP | IP-AP | NP-AP 1 | NP-AP 2 | NP-AP 3 | NP-AP 4 | First-choice AB | Second-choice AB | AB in allergic patient | Duration (days)* | Year and reference | Evidence level 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| North America | |||||||||||||
| USA | EN | 9.3 | 25.4 | 35.7 | 67.3 | 29.2 | 96.6 | Penicillin VK | Amoxicillin | Erythromycin | – | 1996 20 | D |
| USA | GP | 23.2 | 51.8 | 35.1 | 61.6 | 62.5 | 94.9 | Penicillin VK | Amoxicillin | Erythromycin | – | 1996 20 | D |
| USA | EN | 3.5 | 13.3 | 18. | 53.9 | 11.9 | 99.2 | Penicillin VK | Clindamycin | Clindamycin | 7.6 ± 1.7 | 2002 9 | D |
| USA | PD | 2 | 32 | 42 | 68 | 39 | 99 | Amoxicillin | Penicillin VK | – | 2013 19 | D | |
| Europe | |||||||||||||
| UK | GP | 12.5 | 69 | 26 | 90 | Amoxicillin | Penicillin VK | Metronidazole | 5 (3–10) | 2000 21 | D | ||
| Turkey | GP | 100 | 83.3 | – | – | Ampicillin | Amoxicillin | – | – | 2000 29 | D | ||
| UK | GP | 74 | – | 23.2 | – | 75 | 100 | Amoxicillin | Amoxicillin/metronidazole | Erythromycin | – | 2001 23 | D |
| UK | GP | 39 | 44.4 | 68.8 | – | 84.8 | Amoxicillin | Metronidazole | – | – | 2008 22 | D | |
| Spain | EN | 11.4 | 28.6 | 14.3 | 52.9 | 21.4 | 94.3 | Amoxicillin | Metronidazole/spiramicyn | Clindamycin | 6.8 ± 1.8 | 2009 26 | D |
| Belgium | GP | 5.9 | – | – | – | – | 82.7 | Amoxicillin | Clindamycin | Erythromycin | – | 2009 24 | D |
| Lithuania | GP | 2 | – | – | 60 | – | 84 | Amoxicillin | Penicillin VK | – | 2010 25 | D | |
| Spain | DS | 31.5 | 54.3 | 30.7 | 70.9 | 59.8 | 94.5 | Amoxicillin | Clindamycin | Clindamycin | 7.0 ± 1.0 | 2010 27 | D |
| Turkey | GP | 21 | 21.7 | 41 | Amoxicillin | Clindamycin | Clindamycin | 2013 27 | D | ||||
| Croatia | GP | 46 | – | – | – | 85 | Amoxicillin | Clindamycin | Clindamycin | 6.4 ± 1.6 | 2015 30 | D | |
| Asia | |||||||||||||
| Kuwait | GP | 19.6 | 46.4 | 25.0 | – | – | 85.1 | Amoxicillin | Penicillin VK | Erythromycin | – | 2004 31 | D |
| Iran | EN | 26.7 | 44.6 | 79.2 | 85.1 | Amoxicillin | Metronidazole | Erythromycin | – | 2007 32 | D | ||
| Iran | GP | 80.6 | 73.1 | 58 | 74.2 | Amoxicillin | Amoxicillin/metronidazole | – | – | 2011 33 | D | ||
| Iran | GP | 25.1 | 77.2 | 32.9 | 75.3 | Amoxicillin | Penicillin VK | Erythromycin | 6–10 | 2011 34 | D | ||
| India | GP | 60.6 | 65.2 | 44.9 | 56.9 | 69.4 | 92.1 | Amoxicillin | Amoxicillin/metronidazole | Erythromycin | 5 | 2013 35 | D |
| India | GP | 37.6 | 71.6 | 38.2 | 59.1 | 46.9 | 90.2 | Amoxicillin | Oxoflacyn/ornidazole | Erythromycin | 4.3 ± 1.3 | 2014 36 | D |
| India | GP | 7.8 | 10.0 | 3.4 | 7.2 | 15.0 | 56.4 | Amoxicillin | Oxoflacyn/ornidazole | Erythromycin | – | 2014 4 | D |
| Saudi Arabia | GP | 27.3 | 42.0 | 23.5 | 59.0 | 46.4 | 77.0 | Amoxicillin | Amoxicillin/metronidazole | Clindamycin | – | 2015 37 | D |
| Pakistan | GP | 21 | – | – | – | – | Amoxicillin | Amoxicillin/metronidazole | – | – | 2015 38 | D | |
| Africa | |||||||||||||
| Yemen | GP | 32 | 66.3 | 72 | 78 | Amoxicillin | Spiramycin | Erythromycin | – | 2006 39 | D | ||
| Oceania | |||||||||||||
| Australia | GP | – | – | 0 | – | 39 | 98 | Amoxicillin | – | Erythromycin | – | 2000 40 | D |
Treatment duration is indicated in different forms by different investigators; (i) mean ± SD, (ii) mean (range), (iii) only mean, (iv) only range.
AB, antibiotic; D, Very Low; DS, dental surgeon; EN, endodontist; GP, general practitioner; IP, irreversible pulpitis, moderate/severe symptoms; IP-AP, irreversible pulpitis with apical periodontitis, moderate/severe symptoms; NP-AP-1, necrotic pulp with apical periodontitis, no swelling, no/mild symptoms; NP-AP-2, necrotic pulp with apical periodontitis, no swelling, moderate/severe symptoms; NP-AP-3, necrotic pulp with apical periodontitis, sinus tract present, no/mild symptoms; including chronic apical periodontitis; NP-AP-4, necrotic pulp with apical periodontitis, swelling, moderate/severe symptoms, including dentoalveolar abscess; P, prescriber; PD, paediatric dentist.
North America
Early surveys to investigate the antibiotic-prescription pattern in the treatment of pulpitis and apical periodontitis were carried out in the United States, most of them amongst endodontists. Dorn et al.17 carried out several surveys analysing the use of antibiotics by diplomates of the American Board of Endodontists in the treatment of endodontic infections. Swelling and lack of drainage through the canal were the main reasons to prescribe antibiotics, with necrotic pulp with diffuse swelling and no drainage being the condition for which the highest percentage of antibiotics were prescribed (87.6%)17. A decade later, in 1990, antibiotic-prescription habits could be broken down into three categories: prescription of antibiotics for vital pulps (3.5–13.7% of dentists); prescription of antibiotics when the pulp is non-vital and there is no swelling (33% of dentists); and prescription of antibiotics for non-vital pulps with swelling (the largest percentage, 60.5–88.2% of dentists)18. Retrospectively, in 2002, the prescribing habits of active members of the American Association of Endodontists (AAE) were newly analysed, and it was concluded that most of its members were selecting the most suitable antibiotic for the treatment of orofacial infections, but still many were prescribing antibiotics inappropriately.
The antibiotic prescribing habits of paediatric dentists were also studied by analysing the surveys of 4,636 members of the American Academy of Pediatric Dentistry19. A trend was found toward overprescription of antibiotics for the following conditions: irreversible pulpitis, with (32%) and without (42%) vital pulp; and localised dentoalveolar abscess, with (68%) and without (39%) draining fistula.
In the United States, the most frequently prescribed antibiotic is penicillin VK, which is the first-choice antibiotic in 69% of dentists20. Only 28% of American endodontists prescribed amoxicillin9. Erythromycin20 and clindamycin9 were prescribed in patients allergic to penicillin.
Europe
Several surveys have studied the pattern of antibiotic prescription in the treatment of endodontic diseases amongst European dentists. In a survey carried out amongst British general dental practitioners providing National Health Service (NHS) treatment21, more than 95% of dentists prescribed antibiotics for spreading infections; and some dentists (12.5%) prescribed antibiotics for acute pulpitis, either before (69%) or after (23%) the drainage of acute abscesses21. In a later study, Tulip and Palmer22 found that 39% of dentists prescribed antibiotics for pulpitis, 44.4% when apical periodontitis was evident, 68.8% for apical periodontitis with no swelling and 84.8% for acute apical abscess. Dailey and Martin23, studying the prescription of antibiotics in dental emergencies, concluded that in 75% of cases, antibiotics were inappropriately prescribed.
Mainjot et al.24 analysed antibiotic prescribing in dental practice in Belgium. Antibiotic prescriptions were distributed as follows: in the absence of fever (92.2%); for periapical abscess (63.3%); without any local treatment (54.2%); and for pulpitis (4.3%). Among Lithuanian dentists, more than 60% of the respondents reported prescribing antibiotics for symptomatic apical periodontitis25. The majority of the respondents (84%) reported symptomatic apical periodontitis with periostitis as being a clear indication for the prescription of antibiotics. Approximately 2% of the respondents reported prescribing antibiotics for symptomatic pulpitis. A correlation was observed between the duration of professional activity and prescription of antibiotics. The authors concluded that Lithuanian dentists tended to overprescribe antibiotics for cases of pulpitis and apical periodontitis.
Rodríguez-Nuñez et al.26 studied the prescription habits of antibiotics by Spanish endodontists. For irreversible pulpitis, 40% of respondents prescribed antibiotics. For necrotic pulp, acute apical periodontitis and no swelling, 53% of the respondents prescribed antibiotics. Almost 22% of the professionals prescribed antibiotics for necrotic pulps with chronic apical periodontitis and a sinus tract. Some endodontists were prescribing antibiotics unnecessarily to treat minor infections. Segura-Egea et al.27 analysed the use of antibiotics amongst Spanish oral surgeons. Respondents prescribed antibiotics for irreversible pulpitis (86%) and for necrotic pulp, acute apical periodontitis and no swelling (71%). For necrotic pulps with chronic apical periodontitis and a sinus tract, nearly 60% of respondents prescribed antibiotics. Some oral surgeons also prescribed antibiotics inappropriately. Kaptan et al.28 studied the antibiotic-prescription pattern in the treatment of dental emergencies in Turkey: 22% of dentists prescribed antibiotics for patients with acute apical periodontitis, and 41% of dentists prescribed antibiotics for patients with acute apical abscess. A high percentage of Turkish dentists (74.4%) were prescribing antibiotics unnecessarily29. In Croatia30, antibiotics were prescribed in 46% of cases of pulpitis and in 80% of cases diagnosed as acute apical abscess.
Amoxicillin, alone or in combination with clavulanic acid, is the preferred prescribed antibiotic in endodontic infections in all surveys carried out in Europe22., 24., 25., 27., 28., 30.. In Belgium, 82% of all prescriptions were for amoxicillin, amoxicillin+clavulanic acid and clindamycin24. Among Lithuanian dentists, amoxicillin was the antibiotic most preferred during endodontic treatment, followed by amoxicillin+clavulanic acid25. However, an increase in the prescription of penicillin and a decrease in prescribing amoxicillin and amoxicillin+clavulanic acid regarding the increasing age of respondents was reported29. In Spain, amoxicillin was the first-choice antibiotic for 86% of respondents26, followed by metronidazole+spiramycin (8%) and clindamycin (4%). In penicillin-allergic patients, clindamycin 300 mg was the first drug of choice (63%), followed by metronidazole+spiramycin (24%). Similarly, 90% of oral surgeons selected amoxicillin as the first-choice antibiotic and prescribed clindamycin 300 mg (65%) for penicillin-allergic patients27. Kaptan et al.28, in a survey carried out in Turkey, found that 62% of dentists prescribed amoxicillin+clavulanate and 47% prescribed amoxicillin alone. Thirteen years previously, a study indicated that ampicillin was the first-choice antibiotic prescribed by Turkish dentists29. In the UK, the principal antibiotic prescribed in endodontic infections for both adult and child patients was amoxicillin21., 23..
Asia
Salako et al.31 analysed the pattern of antibiotic prescription for dental management in Kuwait. Amongst respondent dentists, 90% prescribed antibiotics when the patient shows evidence of systemic involvement, such as fever and gross or diffuse facial swelling. Sixty per cent reported that they would prescribe antibiotics when endodontic infection is associated with difficulty in swallowing and 50% reported that they would prescribe antibiotics when a patient shows localised fluctuant swelling without any systemic involvement.
Three studies have analysed the patterns of antibiotic prescription amongst Iranian dentists. Amongst the members of the Iranian Association of Endodontists32, a high percentage of responders prescribed antibiotic for fever (78.2%) and diffuse swelling (85.1%), but in some instances, such as in acute pulpitis (26.7%) and chronic periapical lesions (79.2%), antibiotics were inappropriately prescribed. Amongst Iranian general practitioners, Navabizadeh et al.33 found that only 29% of dentists had full knowledge of antibiotic-prescription guidelines for endodontic diseases. However, Vessal et al.34 found that more than 40% of general dentists prescribed antibiotics for problems for which antibiotics were not required, according to good practice guidelines.
The pattern of antibiotics prescription in endodontic diseases in India has also been analysed by several investigators. Kumar et al.35 determined the antibiotic-prescribing habits for pulpal and peri-apical pathology among dentists in Hyderabad, India. The total percentage of dentists who prescribed antibiotics for endodontic management was 68.5%. The most common indication for antibiotics was a necrotic pulp with acute apical periodontitis with swelling and moderate/severe preoperative symptoms (92.1%).
A survey carried out amongst Indian oral health-care providers36 revealed that most prescribed antibiotics for irreversible pulpitis and acute apical periodontitis (72%) and necrotic pulp, acute apical periodontitis and no swelling (59%). The authors concluded that 92% of the oral health-care providers overprescribed antibiotics. Jayadev et al.4 studied the pattern of antibiotic prescription for pulpal and peri-apical pathologies among Indian dentists. Of the respondents to the survey, 44% stated that they would prescribe medication for elevated body temperatures and evidence of systemic involvement, while 42.8% would prescribe medication for non-clinical factors, such as unsure diagnosis. Necrotic pulp with acute apical periodontitis with swelling present and moderate-to-severe preoperative symptoms was the most common condition identified for antibiotic therapy (56%). Fifty-five per cent of dentists would not prescribe an antibiotic and analgesic after root-canal treatment.
Iqbal37 evaluated the pattern of antibiotic prescription of dentists for endodontic infections in northern Saudi Arabia. Amongst respondents, 77% prescribed antibiotics for necrotic pulp with acute apical periodontitis when swelling and moderate or severe preoperative symptoms were present, and 59% prescribed antibiotics when no swelling was present. In patients with irreversible pulpitis with moderate-to-severe symptoms, 27.3% of respondents stated that they would prescribe antibiotics. Tanwir et al.38 recently examined the pattern of antibiotic and analgesic prescriptions per diagnosis by dentists in Karachi, Pakistan. Caries and pulpitis were the most common diagnoses (31%), for which 21% were prescribed antibiotics.
Regarding the most frequently prescribed antibiotics in Asian countries, Iranian general dental practitioners prescribed amoxicillin 500 mg capsules as the drug of choice for endodontic infections33. Amongst Indian dentists, the first antibiotic of choice in patients with no medical allergies is amoxicillin, followed by oxoflacyn/ornidazole4., 36. and amoxicillin+metronidazole35. The drug of choice in patients allergic to penicillin is erythromycin4., 35., 36.. Amoxicillin, administered alone or with clavulanic acid or metronidazole, was the drug of choice in Saudi Arabia37 and clindamycin was the first choice of drug in allergic patients. In Pakistan, amoxicillin and metronidazole were the most common antibiotics prescribed38.
Africa
Only one study carried out in Africa has been found to fulfill the criteria for inclusion in this review. This study assessed the pattern of antibiotics prescribed by general dentists in Yemen39. Higher percentages of overprescription of antibiotics were found: 84% of dentists prescribed an antibiotic for patients without a clinical indication, such as pulpitis (32%), acute apical periodontitis without swelling (66.3%) and chronic apical periodontitis (72%). Amoxicillin and spiramycin were the first- and second-choice antibiotics, respectively, prescribed for endodontic diseases.
Australia
Only one study that assessed antibiotic-prescribing habits in Australia was found. Jaunay et al.40 analysed the prescribing habits of South Australian general dental practitioners in different clinical situations related to periapical pathology. In patients with localised infection, 39% of dentists prescribed an antibiotic, and 28% of dentists prescribed antibiotics for the treatment of a draining sinus. Although dentists knew the appropriate guidelines for antibiotic prescription, they had a tendency toward overprescription. The first-choice antibiotic in the management of endodontic diseases was amoxicillin. The alternative in allergic patients was erythromycin.
South America
Scarce data are available about the prescription of antibiotics by dentists in South American countries. In Brazil, a survey analysed the prescription pattern of systemic antibacterial and analgesic/anti-inflammatory medication by dentists, without reference to endodontic diseases11. Most of the Brazilian general dental practitioners (50.6%) prescribed amoxicillin as the drug of choice and phenoxymethylpenicillin (28%) as the second drug of choice. Erythromycin was the choice in allergic patients.
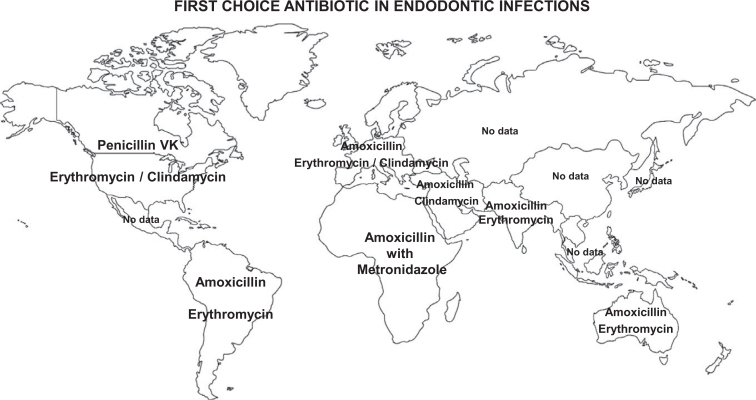
Figure 2 summarises the first-choice antibiotics worldwide in the treatment of endodontic infections in non-penicillin-allergic and penicillin-allergic patients.
Figure 2.
First-choice antibiotics used worldwide for the treatment of endodontic infections in non-penicillin-allergic and penicillin-allergic patients. Two antibiotics are given for each country; the upper one is that given to non-penicillin-allergic patients and the lower one is that given to penicillin-allergic patients. There are no data from China, Indonesia, Brazil, Bangladesh, Russia, Japan and Mexico.
Discussion
One of the main findings of this review is that dentists are overprescribing antibiotics in the management of endodontic infections. A non-indicated condition for prescription of antibiotics in systemically healthy patients is localised swelling. However, in most regions for which data are available, such as North America9., 21., 22., 23., 26., 27., 28., 29., Asia31., 32., 33., 34., 35., 36., 37., 38., Africa39 and Australia40, high percentages of dentists prescribed antibiotics for this condition. It is necessary to improve worldwide prescribing habits of antibiotics in the treatment of endodontic infections. Moreover, educational initiatives must be developed to encourage the coherent and proper use of antibiotics in these conditions21.
Another important finding is that there are scarce or no data about the antibiotic-prescription patterns of dentists in many countries41, some with large populations (such as China, Indonesia, Brazil, Bangladesh, Russia, Japan and Mexico). In these countries, adequate surveys to determine the antibiotic-prescription pattern of dentists in the treatment of endodontic infections should be encouraged.
When there is evidence of systemic involvement and gross, rapid and diffuse spread of infection, antibiotics must be prescribed42. However, most chronic or even acute endodontic infections can be successfully managed by root-canal system disinfection, which eliminates the source of infection, followed by drainage of the abscess without the need for antibiotics27. The dental pulps of patients who have irreversible pulpitis with moderate-to-severe symptoms, with or without an acute apical periodontitis component, can still be vital43. These patients have no signs of systemic involvement and antibiotics are not indicated, but a high percentage of dentists still prescribed antibiotics in these situations18., 24., 31., 44..
A recently published systematic review analysed the evidence available on antibiotic usage for endodontic infections and pain. It was concluded that the best available clinical evidence does not support the prescription of antibiotics for treatment of endodontic diseases unless the spread of infection is systemic, the patient is febrile, or both45. Thus, in cases of necrotic pulp with acute or chronic apical periodontitis, with no swelling and moderate/severe symptoms, antibiotic use is not indicated. The proper treatment in these cases should be limited to endodontic treatment, with debridement of the root-canal space and analgesics. Usually, a correct diagnosis, together with effective root-canal treatment, will be sufficient to reduce the microbial load to allow healing45. However, in this situation, again, a high percentage of dentists prescribe antibiotics4., 17., 18., 27., 36., 38.. The mere presence of a sinus tract, in cases of asymptomatic necrotic pulp with chronic apical abscess, is not an indication for antibiotics because there is no systemic involvement42. The proper treatment of an uncomplicated abscesses is effective drainage and removal of the cause. Nevertheless, prescription of antibiotics for drainage of an abscess related to a tooth has increased two-fold between 1998 and 20061., 41.. However, antibiotics would be indicated in patients with poor health or in immunocompromised patients, when the sinus tract does not disappear or the patient develops a flare up with systemic involvement27. It can be interpreted that systemic involvement is present in patients with necrotic pulp, acute apical periodontitis (abscess), swelling and moderate-to-severe symptoms. In such cases, root-canal treatment, incision and drainage must be complemented with antibiotics. Most dentists (87–99%) prescribe antibiotics appropriately in this situation9., 17., 18., 24., 26., 27., 36., 37.. Oral infections with fever, lymphadenopathy and trismus, or facial cellulitis with or without dysphagia, are serious diseases that should be treated by antibiotics because of the possibility of spread of infection via lymph and blood circulation41.
Regarding the prescribed antibiotics, amoxicillin is the first-choice drug in the treatment of endodontic infections in most countries4., 24., 26., 27., 31., 36., 37., 41.. Amoxicillin represents a synthetic improvement of the original penicillin molecule, being readily absorbed when it is taken with food and resistant to damage from stomach acid46. Moreover, compared with penicillin, amoxicillin has broader spectrum of effectiveness against the cell wall of Gram-negative bacteria, being able to last a bit longer as a result of its resistance to stomach acid46. Amoxicillin+clavulanic acid, because of its broad spectrum, low incidence of resistance, pharmacokinetic profile, tolerance and dosage, is one of the antibiotics recommended for the treatment of odontogenic infections47. Nevertheless, the broad spectrum of amoxicillin is probably more than is required for the treatment of apical periodontitis. The AAE claim that its use in a healthy individual may contribute to the global antibiotic-resistance problem48. This could be the reason why, in the United States, penicillin is the first-choice antibiotic in the treatment of endodontic infections9., 17., 18.. Penicillin is a narrow-spectrum antibiotic that is effective against aerobic Gram-negative cocci and anaerobes. Penicillin has two main drawbacks: its poor absorption from the intestinal tract, meaning that more than 50% of an oral dose is wasted; and its short-acting effect, with half of the amount circulating being removed every half hour46.
Metronidazole has been suggested as a supplemental medication for amoxicillin48 because of its excellent activity against anaerobes. In Europe and the Middle East, metronidazole is the second-choice antibiotic in the treatment of endodontic infections27., 31., 37., and in Asia and Africa the combination amoxicillin+metronidazole is the first-choice drug4., 38..
When a patient is allergic to penicillins, the first drug of choice varies throughout the world. In Spain26., 27. and the United States the first drug of choice is clindamycin9, an antibiotic active against oral anaerobes and facultative bacteria. However, high doses of clindamycin increase the probability of serious side effects, such as pseudomembranous colitis49 and neutropenia50. On the other hand, in Belgium24, the Middle East31 and Asia4., 35. the first-choice antibiotic in penicillin-allergic patients is the macrolide erythromycin; the spectrum of activity of erythromycin against bacteria is comparable with that of penicillin46. In Canada, although there are no data on antibiotic-prescribing patterns in patients with endodontic disease, it has been found that antibiotics prescribed after dental treatment primarily were penicillins13, and that erythromycin13 was prescribed to patients allergic to penicillin.
Since the 1970s, the problem of antibiotic prescription in dentistry and, specifically in endodontics, has been analysed using surveys. The survey instrument has historically been successful in obtaining pertinent information. Specifically, some surveys have been designed to collect information relative to the patient's conditions for which antibiotics were prescribed and the types of antibiotics used. Nevertheless, in these surveys, the overall response rate is not particularly high, ranging from 30% to 45%51. Taking into account that most of the studies included in this review are based on these types of surveys, the pattern of antibiotics prescription from each geographical area may not be well represented by the results of one or a few survey-based studies.
For several years, there have been efforts to develop new antibacterials that are effective against resistance. The concept of anticipation resistance is now emerging. Computational algorithms and experimental evolution could aid in predicting antimicrobial-resistance patterns, thus improving the design of antimicrobial drugs. Computationally predicting drug-resistance mutations early in the discovery phase would be an important breakthrough in antibiotic development.
In conclusion, amoxicillin is the drug of choice for endodontic infections in most countries, and clindamycin and erythromycin are the drugs of choice in patients allergic to penicillin. Dentists worldwide prescribe antibiotics for conditions for which they are not indicated, such as pulpitis. There is overprescription of antibiotics in the management of endodontic infections. It is therefore necessary to amend antibiotic-prescribing habits in the treatment of endodontic infections, as well as to introduce educational initiatives to encourage the coherent and proper use of antibiotics in these conditions.
Acknowledgements
There is no acknowledgement and financial support.
Conflict of interest
The authors deny any conflicts of interest.
References
- 1.Lewis MA. Why we must reduce dental prescription of antibiotics: European Union antibiotic awareness day. Br Dent J. 2008;205:537–538. doi: 10.1038/sj.bdj.2008.984. [DOI] [PubMed] [Google Scholar]
- 2.Sedgley CM, Lee EH, Martin MJ, et al. Antibiotic resistance gene transfer between Streptococcus gordonii and Enterococcus faecalis in root canals of teeth ex vivo. J Endod. 2008;34:570–574. doi: 10.1016/j.joen.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Pallasch TJ. Global antibiotic resistance and its impact on the dental community. J Cal Dent Assoc. 2000;28:215–233. [PubMed] [Google Scholar]
- 4.Jayadev M, Karunakar P, Vishwanath B, et al. Knowledge and pattern of antibiotic and nonnarcotic analgesic prescription for pulpal and periapical pathologies – a survey among dentists. J Clin Diagn Res. 2014;8:ZC10–ZC14. doi: 10.7860/JCDR/2014/9645.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberoi SS, Dhingra C, Sharma G, et al. Antibiotics in dental practice: how justified are we. Int Dent J. 2015;65:4–10. doi: 10.1111/idj.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siqueira JF, Jr, Rôças IN. Exploiting molecular methods to explore endodontic infections: part 2 – redefining the endodontic microbiota. J Endod. 2004;31:488–496. doi: 10.1097/01.don.0000157990.86638.49. [DOI] [PubMed] [Google Scholar]
- 7.Siqueira JF, Jr, Rôças IN, Silva MG. Prevalence and clonal analysis of Porphyromonas gingivalis in primary endodontic infections. J Endod. 2008;34:1332–1336. doi: 10.1016/j.joen.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Mohammadi Z. Systemic, prophylactic and local applications of antimicrobials in endodontics: an update review. Int Dent J. 2009;59:175–186. [PubMed] [Google Scholar]
- 9.Yingling NM, Byrne BE, Hartwell GR. Antibiotic use by members of the American Association of Endodontists in the year 2000: report of a national survey. J Endod. 2002;28:396–404. doi: 10.1097/00004770-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Centre for Evidence Based Medicine . University of Oxford, Medical Sciences Division; 2005. Critical Appraisal for Therapy Articles. Available at: http://www.cebm.net/wp-content/uploads/2014/04/RCT_Appraisal_sheets_2005_English.doc. [Google Scholar]
- 11.Castilho L, Paixao HH, Perini E. Prescription patterns of drugs of systemic use by dentists. Rev Saúde Pública. 1999;33:287–294. doi: 10.1590/s0034-89101999000300010. [DOI] [PubMed] [Google Scholar]
- 12.Roy KM, Bagg J. Antibiotic prescribing by general dental practitioners in the Greater Glasgow Health Board, Scotland. Br Dent J. 2000;188:674–676. doi: 10.1038/sj.bdj.4800574. [DOI] [PubMed] [Google Scholar]
- 13.Epstein JB, Chong S, Nhu DL. A survey of antibiotic use in dentistry. J Am Dent Assoc. 2000;131:1600–1609. doi: 10.14219/jada.archive.2000.0090. [DOI] [PubMed] [Google Scholar]
- 14.Demirbas F, Gjermo PE, Preus HR. Antibiotic prescribing practices among Norwegian dentists. Acta Odontol Scand. 2006;64:355–359. doi: 10.1080/00016350600844394. [DOI] [PubMed] [Google Scholar]
- 15.Al-Haroni M, Skaug N. Incidence of antibiotic prescribing in dental practice in Norway and its contribution to national consumption. J Antimicrob Chemother. 2007;59:1161–1166. doi: 10.1093/jac/dkm090. [DOI] [PubMed] [Google Scholar]
- 16.Marra F, George D, Chong M, et al. Antibiotic prescribing by dentists has increased: why? J Am Dent Assoc. 2016;147:320–327. doi: 10.1016/j.adaj.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Dorn SO, Moodnik RM, Feldman MJ, et al. Treatment of the endodontic emergency: a report based on a questionnaire. Part I. J Endod. 1977;3:94–100. doi: 10.1016/S0099-2399(77)80202-1. [DOI] [PubMed] [Google Scholar]
- 18.Gatewood RS, Himel VT, Dorn SO. Treatment of the endodontic emergency: a decade later. J Endod. 1990;16:284–291. doi: 10.1016/S0099-2399(06)81631-6. [DOI] [PubMed] [Google Scholar]
- 19.Sivaraman SS, Hassan M, Pearson JM. A national survey of pediatric dentists on antibiotic use in children. Pediatr Dent. 2013;35:546–549. [PubMed] [Google Scholar]
- 20.Whitten BH, Gardiner DL, Jeannsonne BG, et al. Current trends in endodontic treatment: report of a national survey. J Am Dent Assoc. 1996;127:1333–1341. doi: 10.14219/jada.archive.1996.0444. [DOI] [PubMed] [Google Scholar]
- 21.Palmer NA, Pealing R, Ireland RS, et al. A study of therapeutic antibiotic prescribing in National Health Service general dental practice in England. Br Dent J. 2000;188:554–558. doi: 10.1038/sj.bdj.4800538. [DOI] [PubMed] [Google Scholar]
- 22.Tulip DE, Palmer NO. A retrospective investigation of the clinical management of patients attending an out of hours dental clinic in Merseyside under the new NHS dental contract. Br Dent J. 2008;205:659–664. doi: 10.1038/sj.bdj.2008.1044. discussion 648. [DOI] [PubMed] [Google Scholar]
- 23.Dailey YM, Martin MV. Are antibiotics being used appropriately for emergency dental treatment? Br Dent J. 2001;191:391–393. doi: 10.1038/sj.bdj.4801190. [DOI] [PubMed] [Google Scholar]
- 24.Mainjot A, D'Hoore W, Vanheusden A, et al. Antibiotic prescribing in dental practice in Belgium. Int Endod J. 2009;42:1112–1117. doi: 10.1111/j.1365-2591.2009.01642.x. [DOI] [PubMed] [Google Scholar]
- 25.Skučait≐ N, Pečiulien≐ V, Manelien≐ R, et al. Antibiotic prescription for the treatment of endodontic pathology: a survey among Lithuanian dentists. Medicina (Kaunas) 2010;46:806–813. [PubMed] [Google Scholar]
- 26.Rodriguez-Nuñez A, Cisneros-Cabello R, Velasco-Ortega E, et al. Antibiotic use by members of the Spanish endodontic society. J Endod. 2009;35:1198–1203. doi: 10.1016/j.joen.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Segura-Egea JJ, Velasco-Ortega E, Torres-Lagares D, et al. Pattern of antibiotic prescription in the management of endodontic infections among Spanish oral surgeons. Int Endod J. 2010;43:342–350. doi: 10.1111/j.1365-2591.2010.01691.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaptan RF, Haznedaroglu F, Basturk FB, et al. Treatment approaches and antibiotic use for emergency dental treatment in Turkey. Ther Clin Risk Manag. 2013;9:443–449. doi: 10.2147/TCRM.S52009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandemir S, Ergül N. Grievances in cases using antibiotics due to orodental problems and assessment of the need for antibiotics. Int Dent J. 2000;50:73–77. doi: 10.1002/j.1875-595x.2000.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 30.Peric M, Perkovic I, Romic M, et al. The pattern of antibiotic prescribing by dental practitioners in Zagreb, Croatia. Cent Eur J Public Health. 2015;23:107–113. doi: 10.21101/cejph.a3981. [DOI] [PubMed] [Google Scholar]
- 31.Salako NO, Rotimi VO, Adib SM, et al. Pattern of antibiotic prescription in the management of oral diseases among dentists in Kuwait. J Dent. 2004;32:503–509. doi: 10.1016/j.jdent.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Kakoei S, Raoof M, Baghaei F, et al. Pattern of antibiotic prescription among dentists in Iran. Iran Endod J. 2007;2:19–23. [PMC free article] [PubMed] [Google Scholar]
- 33.Nabavizadeh MR, Sahebi S, Nadian I. Antibiotic prescription for endodontic treatment: general dentist knowledge + practice in Shiraz. Iran Endod J. 2011;6:54–59. [PMC free article] [PubMed] [Google Scholar]
- 34.Vessal G, Khabiri A, Mirkhani H, et al. Study of antibiotic prescribing among dental practitioners in Shiraz, Islamic Republic of Iran. East Mediterr Health J. 2011;17:763–769. [PubMed] [Google Scholar]
- 35.Kumar KP, Kaushik M, Kumar PU, et al. Antibiotic prescribing habits of dental surgeons in Hyderabad City, India, for pulpal and periapical pathologies: a survey. Adv Pharmacol Sci. 2013;2013:537385. doi: 10.1155/2013/537385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg AK, Agrawal N, Tewari RK, et al. Antibiotic prescription pattern among Indian oral healthcare providers: a cross-sectional survey. J Antimicrob Chemother. 2014;69:526–528. doi: 10.1093/jac/dkt351. [DOI] [PubMed] [Google Scholar]
- 37.Iqbal A. The attitudes of dentists towards the prescription of antibiotics during endodontic treatment in north of Saudi Arabia. J Clin Diagn Res. 2015;9:ZC82–ZC84. doi: 10.7860/JCDR/2015/13718.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanwir F, Marrone G, Tariq A, et al. Diagnosis and prescribing pattern of antibiotics and painkillers among dentists. Oral Health Prev Dent. 2015;13:75–83. doi: 10.3290/j.ohpd.a32341. [DOI] [PubMed] [Google Scholar]
- 39.Al-Haroni M, Skaug N. Knowledge of prescribing antimicrobials among Yemeni general Dentists. Acta Odontol Scand. 2006;64:274–280. doi: 10.1080/00016350600672829. [DOI] [PubMed] [Google Scholar]
- 40.Jaunay T, Sambrook P, Goss A. Antibiotic prescribing practices by South Australian general dental practitioners. Aust Dent J. 2000;45:179–186. doi: 10.1111/j.1834-7819.2000.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 41.Dar-Odeh NS, Abu-Hammad OA, Al-Omiri MK, et al. Antibiotic prescribing practices by dentists: a review. Ther Clin Risk Manag. 2010;6:301–306. doi: 10.2147/tcrm.s9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeitoun IM, Dhanarajani PJ. Cervical cellulitis and mediastinitis caused by odontogenic infections: report of two cases and review of literature. J Oral Maxillofac Surg. 1995;53:203–208. doi: 10.1016/0278-2391(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 43.Estrela C, Guedes OA, Silva JA, et al. Diagnostic and clinical factors associated with pulpal and periapical pain. Braz Dent J. 2011;22:306–311. doi: 10.1590/s0103-64402011000400008. [DOI] [PubMed] [Google Scholar]
- 44.Keenan JV, Farman AG, Fedorowicz Z, et al. Antibiotic use for irreversible pulpitis. Cochrane Database Syst Rev. 2010;5:D004969. doi: 10.1002/14651858.CD004969.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Aminoshariae A, Kulild J. Evidence-based recommendations for antibiotic usage for endodontic infections and pain: a systematic review. J Am Dent Assoc. 2016;147:186–191. doi: 10.1016/j.adaj.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodontal Res. 2002;37:389–398. doi: 10.1034/j.1600-0765.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 47.Kuriyama T, Williams DW, Yanagisawa M, et al. Antimicrobial susceptibility of 800 anaerobic isolates from patients with dentoalveolar infection to 13 oral antibiotics. Oral Microbiol Immunol. 2007;22:285–288. doi: 10.1111/j.1399-302X.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 48.American Association of Endodontists (AAE) AAE Endodontics Colleagues for Excellence; 1999. Prescription for the future: responsible use of antibiotics in endodontic therapy; pp. 1–8. [Google Scholar]
- 49.Trexler MF, Fraser TG, Jones MP. Fulminant pseudomembranous colitis caused by clindamycin phosphate vaginal cream. Am J Gastroenterol. 1997;92:2112–2113. [PubMed] [Google Scholar]
- 50.Bubalo JS, Blasdel CS, Bearden DT. Neutropenia after singledose clindamycin for dental prophylaxis. Pharmacotherapy. 2003;23:101–103. doi: 10.1592/phco.23.1.101.31920. [DOI] [PubMed] [Google Scholar]
- 51.Lee M, Winkler J, Hartwell G, et al. Current trends in endodontic practice: emergency treatments and technological armamentarium. J Endod. 2009;35:353–359. doi: 10.1016/j.joen.2008.10.007. [DOI] [PubMed] [Google Scholar]