Abstract
The gut microbiome is an important contributor to human health, shaped by many endogenous and exogenous factors. The gut microbiome displays sexual dimorphism, suggesting influence of sex hormones, and also has been shown to change with aging. Yet, little is known regarding the influence of menopause – a pivotal event of reproductive aging in women – on the gut microbiome. Here, we summarize what is known regarding the interrelationships of female sex hormones and the gut microbiome, and review the available literature on menopause, female sex hormones, and the gut microbiome in humans. Taken together, research suggests that menopause is associated with lower gut microbiome diversity and a shift toward greater similarity to the male gut microbiome, however more research is needed in large study populations to identify replicable patterns in taxa impacted by menopause. Many gaps in knowledge remain, including the role the gut microbiome may play in menopause-related disease risks, and whether menopausal hormone therapy modifies menopause-related change in the gut microbiome. Given the modifiable nature of the gut microbiome, better understanding of its role in menopause-related health will be critical to identify novel opportunities for improvement of peri- and post-menopausal health and well-being.
Keywords: menopause, gut microbiome, estrobolome, microbial translocation, estrogen, progesterone
Introduction
At current life expectancy, women on average spend approximately three decades of life post-menopause. This significant life chapter is met with elevated risk of age-related chronic diseases, such as diabetes and cardiovascular disease, with risk possibly accelerated by the menopausal transition itself.1–3 Declines in ovarian sex hormones during menopause, particularly estradiol, are credited with directly triggering these health effects. For example, estrogens have many direct actions via estrogen receptor signaling which are beneficial to the cardiovascular system,4 and such estrogen-related cardioprotection is lost after menopause. Menopause-related health outcomes may also be influenced indirectly through the gut microbiome – the community of microorganisms residing in the human gut, which are known to metabolize estrogens and other sex hormones. Menopausal influence on the gut microbiome may have potentially broad consequences, given the diverse actions of gut microbiota in human health. However, relatively little is known regarding the impact of menopause on the gut microbiome and resulting health sequelae. In this review, we summarize the current state of knowledge on the relationship of menopause and the gut microbiome and highlight research gaps and areas for future investigation.
The Menopausal Transition
Menopause is the final stage of reproductive aging in women, typically defined retrospectively after a woman has ceased menstruating for 12 months. During the menopausal transition, which occurs over a span of several years, declines in the number of remaining ovarian follicles lead to decreased and variable production of the ovarian reproductive hormones estradiol and progesterone, and more variable/abnormal menstrual cycles with or without ovulation.5 The declines in ovarian hormones lessen negative feedback on the pituitary, causing elevations in follicle stimulating hormone (FSH), a hallmark of the menopausal transition. Culminating at menopause (ie, the final menstrual period), complete depletion of ovarian follicles leads to loss of ovarian production of estradiol and progesterone, which stay low for the remainder of a woman’s life. After menopause, peripheral tissues (mainly adipose tissue) become the primary producers of estrogens, derived from adrenal androgen precursors.6
The menopausal transition may be accompanied by a number of symptoms ranging in severity woman-to-woman, including vasomotor symptoms (ie, hot flashes), genitourinary symptoms such as vaginal atrophy and dryness, mood and sleep changes, and bone loss.5 Menopausal hormone therapy (HT) with estradiol is effective in alleviating symptoms of menopause.
The Gut Microbiome
Over the past two decades, advances in next-generation sequencing technology have expanded the study of microbial communities to an unprecedented degree.7 Study of the microbes (bacteria, archaea, fungi, and viruses) colonizing the human body, known collectively as the human microbiome, has uncovered extensive taxonomic and functional diversity8 and large variation across body sites and between individuals.9 It is estimated that microbial cells in the human body exist in approximately a 1:1 ratio with human cells, though the number of microbial genes outnumbers human genes at a ratio of ≥100:1, reflecting the vast genetic diversity of the microbiome.10 Research into this new frontier has revealed a significant role for microbes in human health and disease, with much attention focused on the high biomass microbial community of the digestive tract (ie, the gut microbiome). The gut microbiota carry out many functions which can contribute to health homeostasis or disease, including metabolism of dietary components (eg, fiber, amino acids) and endogenous compounds (eg, bile acids, hormones), and biosynthesis of lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria which triggers inflammation.11 The products of the gut microbiota can reach far past the gastrointestinal tract, even influencing the central nervous system via the “gut microbiota-brain axis”, a bi-directional interaction involving metabolic, neuroendocrine, and immune pathways which is important for the homeostatic maintenance of the gastrointestinal and central nervous systems.12,13 Studies in humans have observed correlations of gut microbiome composition with a wide range of conditions and diseases, including obesity, inflammatory bowel disease, colorectal cancer, depression, diabetes, and cardiovascular disease.14 In many instances, reduced diversity of the gut microbiome is indicative of dysbiosis, and associated with Western lifestyle and various disease states.15 Research on the gut microbiome is motivated by a strong translational potential to utilize the gut microbiome as a biomarker of disease,10 as well as the possibility to modulate the gut microbiota to alter the onset or progression of disease.11
Aging and Sexual Dimorphism of the Gut Microbiome
An individual’s gut microbiome composition shifts over the course of their lifetime, with the largest shifts occurring in early life during weaning, when the diversity of the gut microbiome skyrockets upon the transition from breastmilk to solid food.16,17 A shift towards sexual dimorphism of the gut microbiome may occur during puberty and adolescence, since teenage male-female dizygotic twin pairs have greater microbiome dissimilarity than same gendered dizygotic twin pairs, a pattern not observed in infancy,18 and the gut microbiome in girls was shown to shift toward an adult-like state during pubertal progression.19 Post-pubertal sexual dimorphism of the gut microbiome is supported by animal studies: weanling male and female mice are indistinguishable in their gut microbiome composition, but sex differences emerge at puberty and are most apparent in adult mice.20,21 This evidence strongly implicates sex hormones in shaping gut microbiome structure during puberty and onward.
In adulthood, gut microbiome diversity continues to increase with advancing age, which may reflect underlying age-related changes in immunity, mucosal function, diet, metabolism, diseases, and other factors. Gut microbiome diversity appears to plateau around age 40,22 while the composition of the gut microbiota transitions towards a state of uniqueness within each person with increasing age.23 The gut microbiome is also distinct in extremely long-lived individuals (ie, nonagenarians and centenarians) compared with younger adults,17,24 and specific gut microbiome characteristics such as high uniqueness and low Bacteroides dominance have been related to healthy aging and longevity.23 Regarding sexual dimorphism of the gut microbiome in adulthood, several large studies in humans have shown differences between men and women, including higher gut microbiome richness and lower Prevotella abundance in women compared to men.25–30 However, sex differences in aging of the gut microbiome remain under-explored, especially regarding menopause, a significant milestone of reproductive aging in women. Interestingly, one study of aging found that women have higher gut microbiome diversity than men in younger adulthood but not in older adulthood,22 which is suggestive of sex differences in microbiome aging related to menopause.
The Sex Hormone-Gut Microbiome Axis
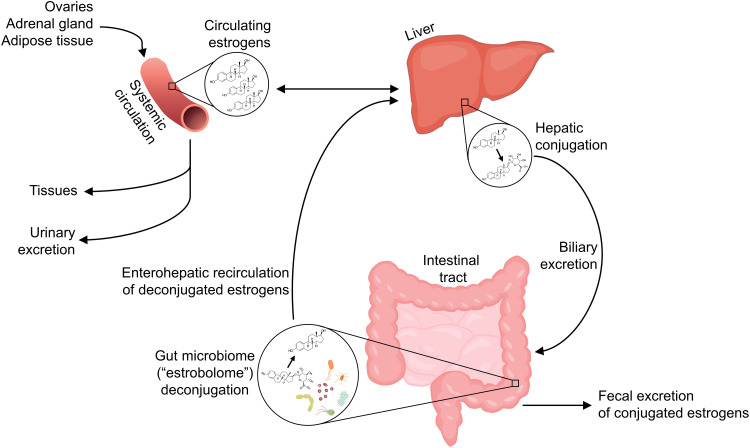
While the sex hormone milieu appears to impact the gut microbiome, resulting in sexual dimorphism, the gut microbiota are also involved in regulating free circulating hormone levels, suggesting a bi-directional relationship. Endogenous estrogens, namely estradiol (predominant in pre-menopause), estrone (predominant in post-menopause), and estriol (predominant in pregnancy), are metabolized in the liver through first-pass irreversible hydroxylation and/or conjugation with glucuronide or sulfate groups. Glucuronide or sulfate conjugation in the liver allows for biliary excretion of estrogens by way of the intestinal tract (ie, fecal excretion). However, some gut microbiota possess the ability to deconjugate estrogens, releasing them for reabsorption into the enterohepatic circulation; through this “recycling” mechanism, the estrogens may again access the systemic circulation and some target tissues31 (Figure 1). The aggregate of bacterial genes capable of deconjugating estrogens is termed the “estrobolome”.32 Though much literature focuses on the gut microbiome and estrogens, other hormones excreted in bile including progesterone33 and androgens34 are similarly deconjugated and recirculated by the gut microbiome. Thus, presumably, sex hormones influence the gut microbiota in part by serving as substrates for microbial metabolism, and in turn the gut microbiota influence sex hormone levels via deconjugation.
Figure 1.
Enterohepatic recirculation of estrogens by the gut microbiome. Estrogens in the systemic circulation, produced by the ovaries, adrenal gland, and adipose or other tissues, undergo first-pass metabolism in the liver, and also may be conjugated with glucuronide or sulfate groups in the liver which facilitates biliary excretion. In the intestinal tract, conjugated estrogens are either excreted in feces, or deconjugated by gut microbiota with β-glucuronidase or sulfatase enzymes, termed the “estrobolome” – this allows estrogens to enter the enterohepatic circulation, and thus subsequently re-enter the systemic circulation and reach other tissues.
Studies of injected radioactively labeled estradiol and estrone in women have shown that, while much of the injected estrogens are excreted in bile (approximately 50%),35,36 a much smaller fraction (eg, 7%35 or 10–15%37) occur in conjugated form in feces, indicating that a significant proportion of estrogens are reabsorbed in the circulation via deconjugation activity of the gut microbiome. Many gut species across major gut phyla, especially Firmicutes and Bacteroidetes, possess β-glucuronidase38,39 and sulfatase40 enzymes, acting in the deconjugation of glucuronide or sulfate groups, respectively. Human gut bacterial β-glucuronidases have been shown in vitro to deconjugate estradiol and estrone glucuronides,41 providing proof-of-principle that human gut microbiota can reactivate estrogens.
Some studies have examined correlations of the gut microbiome with directly measured estrogens or progesterone in humans (Table 1). A series of studies by scientists at the US National Institutes of Health and their collaborators have examined the relationship of the gut microbiome with urinary estrogens. In post-menopausal women (n = 7) and men (n = 25), gut microbiome richness, diversity, and Clostridia abundance were strongly positively related to urinary total estrogens as well as estrogen metabolites.42 Functional activity of fecal β-glucuronidase was positively correlated with urinary estrone, and inversely correlated with fecal total estrogens, suggesting recycling of estrogens by microbial β-glucuronidase activity.42 These associations were not observed in pre-menopausal women (n = 19), possibly because urine samples were collected irrespective of menstrual cycle timing, and thus highly variable in levels of estrogens.42 Another study of 60 post-menopausal women observed a positive association of urinary total estrogens with Ruminococcus,43 which is an anaerobic gram-positive genus involved in complex carbohydrate fermentation to short-chain fatty acids.44
Table 1.
Summary of Studies of Menopause or Sex Hormones and the Gut Microbiome in Women
| First Author Last Name (Year) | Location | Sample Size | Method | Results (Post- vs Pre-Menopause) | Results (Hormones) | Control for Age |
|---|---|---|---|---|---|---|
| Studies of menopause and hormones | ||||||
| Santos-Marcos (2018)47 | Spain | n=17 pre-menopausal women n=20 post-menopausal women n=39 men matched to pre- and post-menopausal women on age, BMI, and nutritional background |
16S rRNA gene sequencing | No difference in α-diversity Difference in β-diversity Post-menopausal microbiome more similar to men than pre-menopausal to men ↑ Firmicutes, Roseburia, Lachnospira ↓ Bilophila, Prevotella, Parabacteroides |
Plasma estradiol: ↑ Gammaproteobacteria, Mixococcales ↓ Prevotellaceae |
None |
| Mayneris-Perxachs (2020)48 | Spain | n=44 pre-menopausal women n=45 post-menopausal women n=42 men |
Shotgun metagenomic sequencing | No difference in α- or β-diversity Post-menopausal microbiome more similar to men than pre-menopausal to men Menopausal differences attenuated among obese 90 differentially abundant taxa in post- vs pre-menopause, including: ↑ Bacteroides sp. CAG:661, Anaerovibrio sp. JC8, Prevotella sp. P6B1, Lactobacillus rhamnosus, Dorea sp. CAG:317, Veillonella dispar, Haemophilus parainfluenzae ↓ Ruminococcus sp. CAG:379, Clostridium neonatale, Acidiphilium sp. CAG: 727, Bifidobacterium angulatum, Alistipes sp. AL-1, Eubacterium sp. CAG:603, Enterococcus faecalis, Blautia sp. CAG:257; pyrimidine and one carbon pool by folate pathways |
Plasma progesterone concentration predicted by microbiome composition (no taxa specified) | Multivariable adjustment |
| Wu (2021)46 | China | n=35 premature ovarian insufficiency (POI) <40 years old n=18 pre-menopausal with normal ovarian function <40 years old |
16S rRNA gene sequencing | No difference in α- or β-diversity ↑ Bacteroidetes, Butyricimonas, Dorea, Lachnobacterium, Sutterella ↓ Firmicutes, Bulleidia, Faecalibacterium |
Serum FSH: ↑ Bacteroidetes ↓ Firmicutes Serum estradiol: ↑ Firmicutes, Faecalibacterium ↓ Bacteroidetes Serum AMH: ↑ Faecalibacterium ↓ Bacteroidetes, Butyricimonas |
None (though age was similar between groups) |
| Peters (2022)50 | U.S. | n=295 pre-menopausal n=1027 post-menopausal n=978 men matched to pre- and post-menopausal women on age, BMI, and Hispanic/Latino background |
Shotgun metagenomic sequencing | ↓ α-diversity Difference in β-diversity Post-menopausal microbiome more similar to men than pre-menopausal to men ↑ Bacteroides sp.Ga6A1, Prevotella marshii, Sutterella wadsworthensis; sulfate transport system pathway ↓ Escherichia coli-Shigella spp., Oscillibacter sp.KLE1745, Akkermansia muciniphila, [Clostridium] lactatifermentans, Parabacteroides johnsonii, Veillonella seminalis; pathogenic bacterial secretion system pathways; β-glucuronidase gene ortholog |
Serum progestin metabolites in post-menopausal women: ↑ [Clostridium] lactatifermentans, Akkermansia muciniphila, β-glucuronidase gene ortholog, aryl-sulfatase gene ortholog ↓ Sulfate transport system Serum progestin metabolites in pre-menopausal women not associated with species |
Multivariable adjustment |
| Studies of menopause only | ||||||
| Zhao (2019)64 | China | n=24 pre-menopausal women n=24 post-menopausal women matched to pre-menopausal women on age and BMI |
Shotgun metagenomic sequencing | ↓ α-diversity Difference in β-diversity ↓ Firmicutes, Ruminococcus, Abiotrophia; pentose phosphate and proline degradation pathways ↑ Glycolysis, lactate consumption, cysteine biosynthesis, and homocysteine, lysine and arginine degradation pathways |
N/A | Matching |
| Peters (2021)65 | U.S. | n=182 post-menopausal HIV+ n=99 pre-menopausal HIV+ n=80 post-menopausal HIV- n=71 pre-menopausal HIV- |
16S rRNA gene sequencing | HIV+: ↑ α-diversity Difference in β-diversity ↑ Enterobacteriales, ASVs from [Ruminococcus] torques, Bacteroides, Parabacteroides distasonis ↓ ASVs from Prevotella copri HIV-: No difference in α- or β-diversity ↑ ASV from Parabacteroides distasonis ↓ ASVs from Shigella, Roseburia inulinivorans, Gemmiger formicilis |
N/A | Multivariable adjustment |
| Studies of hormones only | ||||||
| Flores (2012)42 | U.S. | n=19 pre-menopausal women n=7 post-menopausal women n=25 men |
16S rRNA gene sequencing | N/A | In post-menopausal women and men: Total urinary estrogens: ↑ α-diversity ↑ Clostridia (non-Clostridiales and Ruminococcaceae) Urinary estrone: ↑ Fecal β-glucuronidase activity Fecal total estrogens: ↓ Fecal β-glucuronidase activity In pre-menopausal women: No associations observed |
Multivariable adjustment |
| Fuhrman (2014)43 | U.S. | n=60 post-menopausal women | 16S rRNA gene sequencing | N/A | Total urinary estrogens: ↑ Ruminococcus Ratio of estrogen metabolites to parent estrogens: ↑ α-diversity ↑ Clostridiales, Ruminococcaceae ↓Bacteroides |
Multivariable adjustment |
| Shin (2019)45 | Korea | n=26 pre- and post-menopausal women | 16S rRNA gene sequencing | N/A | Serum estradiol: ↑ α-diversity ↓ Slackia, Butyricimonas |
Excluded taxa correlated with age |
| Mihajlovic (2021)49 | Austria | n=16 pre-menopausal women | 16S rRNA gene sequencing | N/A | Combined oral contraceptive users vs non-users: ↓ α-diversity ↓ Eubacterium, Haemophilus, unclassified Firmicutes ↑ Akkermansia, Barnesiella Luteal vs follicular phase: ↑ Akkermansia, Lactococcus |
Contraceptive users and non-users matched on age |
Notes: ↑ and ↓ indicates enriched or depleted abundance of taxon, respectively, in relation to specified continuous variable or group comparison; italicized text indicates genus or species.
Abbreviation: ASV, amplicon sequence variant.
Other studies have examined the relationship of the gut microbiome with estradiol or progesterone measured in blood. Serum estradiol was associated with increased gut microbial diversity and lower abundance of genera Slackia and Butyricimonas among 26 pre- and post-menopausal women in Korea,45 higher abundance of Firmicutes and Faecalibacterium and lower abundance of Bacteroidetes among 53 women with or without premature ovarian insufficiency (POI) in China,46 and higher abundance of Gammaproteobacteria and lower abundance of Prevotellaceae among 37 pre- and post-menopausal women in Spain.47 A study of 89 pre- and post-menopausal women from Spain reported that gut microbiome composition could significantly predict plasma progesterone levels.48 Among 16 pre-menopausal women from Austria, use of combined hormonal contraceptives, which decreased serum estradiol and progesterone on average, was associated with decreased gut microbiome diversity, lower abundance of genera Eubacterium, Haemophilus and unclassified Firmicutes, and higher abundance of Akkermansia and Barnesiella, controlling for menstrual timing.49 Additionally, Akkermansia and Lactococcus were enriched in the luteal phase (high estradiol and progesterone) vs the follicular phase (low estradiol and progesterone) of the menstrual cycle.49 Lastly, in Hispanic/Latino women in the US, we observed correlations between gut species and serum progestin metabolites in post-menopausal women (n = 192) but not pre-menopausal women (n = 154), and gut microbiome “estrobolome” potential (eg, orthologs of β-glucuronidase and aryl-sulfatase genes) was also positively related to serum progestin metabolites in post-menopausal women,50 suggesting recycling of progesterone by the gut microbiota.
These studies support a bi-directional relationship of sex hormones with the gut microbiome, in which higher levels of estrogens and progesterone promote increased microbial diversity by serving as substrates for a diverse range of species, while at the same time, higher gut microbiome diversity and greater deconjugation activity may promote hormone retention. As post-menopausal women lack ovarian hormone production and have very low levels of estrogens and progesterone, enterohepatic recycling of hormones by the gut microbiota may be an important determinant of systemic estrogens and progesterone levels after menopause.
Menopause and Microbial Translocation
The gut microbiota may exert their influence on human health not only through metabolic activities, but also via microbial translocation, a process in which gut epithelial barrier dysfunction allows microbes and microbial products to translocate from the gut lumen to the systemic circulation.51 In the healthy gastrointestinal tract, several layers of defense prevent microbiota from traversing into the circulation, including mucus and epithelial barriers and resident macrophages. However, under certain conditions of immunodeficiency, pathogenic invasion, or inflammation in the gut, the structural integrity of the gut barrier is damaged and microbial translocation can occur. Microbial translocation leads to systemic immune activation and inflammation, and is implicated in the pathogenesis of many diseases, included inflammatory bowel disease and human immunodeficiency virus (HIV) infection.51
Experimental evidence indicates that estradiol and progesterone maintain the gut barrier and protect from gut injury. In vitro and ex vivo studies have found that female rat intestines are more resistant to shock-induced injury than male rat intestines (reversed by estradiol administration);52 estradiol treatment protects mucus-producing intestinal epithelial cells against oxidant injury;53 estrogen receptor-β signaling maintains colonic epithelial barrier function;54,55 and estradiol and progesterone improve epithelial barrier function in intestinal epithelial cells by upregulating tight junction proteins.56,57 In vivo, ovariectomy has been shown to increase intestinal permeability in mice.58 Observationally, estrogen receptor-β mRNA is reduced in the colon of animal with colitis, and in the colon of patients with inflammatory bowel disease.54 Plasma progesterone levels during human pregnancy are inversely correlated with plasma lipopolysaccharide, a marker of microbial translocation.57,59 Taken together, this evidence suggests that the ovarian hormones estradiol and progesterone are important in limiting intestinal permeability and preventing microbial translocation.
It is well known that estrogens and progesterone are master regulators of the immune system and mucosal barrier in the female reproductive tract, leading to bothersome vaginal symptoms and increased susceptibility to pathogenic infections during menopause.60,61 These hormones may be similarly important in the gastrointestinal tract, with menopause reducing barrier integrity and increasing microbial translocation in the gut as well, though few studies have examined the association of menopause with gut barrier integrity and microbial translocation in humans. A recent report of 65 women from the Study of Women’s Health Across the Nation (SWAN) showed that plasma intestinal fatty acid binding protein (IFAB; a marker of gut epithelial cell function), lipopolysaccharide binding protein (LBP; a marker of microbial translocation), and soluble CD14 (sCD14; a marker of immune activation related to microbial translocation) increased significantly within-woman from pre- to post-menopause, while lower plasma estradiol was associated with higher IFAB and sCD14.62 Though it is difficult to disentangle this finding from changes related to aging, it suggests that microbial translocation increases over the menopausal transition, related to sex hormone changes. In a study of 350 women with HIV, we have observed that plasma sCD14, but not IFAB, is elevated in post-menopausal compared to pre-menopausal women63 – we speculate that the elevation in sCD14 could be related to increased microbial translocation, or to other factors. However, a small study of 37 women found no difference in LPS or LBP by menopause status.47 More research is needed to determine the impact of menopause on gut barrier function and microbial translocation in humans.
Human Studies of Menopause and the Gut Microbiome
A number of cross-sectional human studies have compared gut microbiome composition between post- and pre-menopausal women (Table 1). Most studies accounted for age via matching or multivariable adjustment – a crucial step in mitigating confounding by chronological aging. Some studies also measured female sex hormones to support hormonal underpinnings of menopause-related findings in the gut microbiome. Mayneris-Perxachs et al found greater differences in gut microbiome composition between pre-menopausal women (n = 44) and men (n = 42) than post-menopausal women (n = 45) and men.48 These differences were attenuated when restricting to participants with obesity, perhaps related to estrogen production by excess adipose tissue.48 They observed 90 differentially abundant species between pre- and post-menopausal women adjusting for age and obesity status, including species Bacteroides sp. CAG:661, Prevotella sp. PB61, Dorea sp. CAG:317, and Veillonella dispar (enriched in post-menopause), and Ruminococcus sp. CAG:379 and Clostridium neonatale (depleted in post-menopause).48 In that study, gut microbiome composition was able to predict plasma progesterone concentrations, but specific taxa with high predictive ability were not specified. Santos-Marcos et al similarly found that pre-menopausal women (n = 17) differed more from age-matched men than post-menopausal women (n = 20) from age-matched men; they also reported enrichment of Firmicutes, Roseburia, and Lachnospira and depletion of Bilophila, Prevotella, and Parabacteriodes in post-menopausal compared to pre-menopausal women, however this analysis did not control for age.47 They additionally found that plasma estradiol was related to higher abundance of Gammaproteobacteria, and lower abundance of Prevotellaceae, with lack of adjustment for age.47 In a study of premature ovarian insufficiency (POI), Wu et al observed that women with POI (n = 35) did not differ in gut microbiome diversity from pre-menopausal women with normal ovarian function (n = 18), though women with POI had higher abundance of Bacteroidetes, Butyricimonas, Dorea, Lachnobacterium, and Sutterella, and lower abundance of Firmicutes, Bulleidia, and Faecalibacterium.46 Additionally, serum estradiol was associated with higher abundance of Firmicutes and Faecalibacterium, and lower abundance of Bacteroidetes.46 In a large study of US Hispanics/Latinos, we recently reported that the gut microbiome of post-menopausal women (n = 1027) was less diverse than pre-menopausal women (n = 295), and more similar to that of men than was the pre-menopausal gut microbiome.50 Additionally, we found that post-menopausal women had higher abundance of Bacteroides sp.Ga6A1, Prevotella marshii, and Sutterella wadsworthensis, and lower abundance of Escherichia coli-Shigella spp., Oscillibacter sp.KLE1745, Akkermansia muciniphila, [Clostridium] lactatifermentans, Parabacteroides johnsonii, and Veillonella seminalis. Regarding functional capacity, post-menopausal women were found to have higher levels of the sulfate transport system pathway, and lower levels of pathogenic bacterial secretion system pathways and the β-glucuronidase gene ortholog.50 Using serum metabolomics data available in a subset of participants (n = 154 pre-menopausal and n = 192 post-menopausal), we observed that gut species and functions depleted in menopause, including [Clostridium] lactatifermentans, Akkermansia muciniphila, and the β-glucuronidase gene ortholog, were positively correlated with serum progestin metabolites in post-menopausal women, but not in pre-menopausal women.50
Some studies only compared gut microbiome by menopause status, without measurement of sex hormones. Zhao et al reported lower gut microbiome diversity and differences in gut microbiome composition for post-menopausal (n = 24) compared to pre-menopausal (n = 24) women matched on age and BMI, including lower abundance of Firmicutes, Ruminococcus, and Abiotrophia in post-menopausal women.64 In a study of women with and without HIV, we found that post-menopausal women with HIV (n = 182) had higher gut microbiome diversity, higher abundance of Enterobacteriales, and lower abundance of amplicon sequence variants (ASVs) from Prevotella copri than pre-menopausal women with HIV (n = 99), while post-menopausal women without HIV (n = 80) had higher abundance of an ASV from Parabacteroides distasonis and lower abundance of ASVs from Shigella, Roseburia inulinivorans, and Gemmiger formicilis than pre-menopausal women without HIV (n = 71).65
Heterogeneity of results between studies could be related to small sample sizes in some studies and/or different study populations, as the gut microbiome is well known to vary by geography and lifestyle.18,66,67 However, some patterns of the menopause – gut microbiome relationship are emerging that are also in agreement with estrogens and progesterone – gut microbiome relationships (Table 1). Out of 10 total studies of menopause or female sex hormones and the gut microbiome in humans to date (n = 6 menopause studies with or without hormones; n = 4 hormone only studies), 5 studies found decreased α-diversity in post- vs pre-menopausal women or in women with lower estrogen42,45,49,50,64 and 3 studies observed more similarity to the gut microbiome in men for post- vs pre-menopausal women.47,48,50 Although various gut bacterial taxa were reported to differ by menopause status, some consistent results were observed across studies. For example, multiple studies found lower abundance of Firmicutes46,64 and Ruminococcus43,48,68 (genus or species), and higher abundance of Butyricimonas,45,46 Dorea,46,48 Prevotella,48,50 Sutterella,46,50 and Bacteroides48,50 genera or species, in post- vs pre-menopausal women or in women with lower estrogen. As the functions and health influences of these bacteria are not fully known, it is difficult to yet surmise whether menopause may have a disadvantageous impact on the gut microbiome composition based on available information. Ruminococci are fiber fermenters and producers of short-chain fatty acids, which is considered a beneficial function.69 Reduced abundance of some Ruminococcus species has been observed in patients with Crohn’s disease, though other species are associated with diseases such as diverticulitis and arthritis.44 Dorea, Prevotella, and Sutterella have all been previously associated with obesity in multiple studies.70 Prevotella is a very diverse genus of fiber degraders, which are more abundant in non-Westernized than Westernized populations; research is conflicting on whether Prevotella are beneficial or detrimental to human health, likely related to different strains having differing health effects.71 Similarly, gut Bacteroides can be beneficial or harmful depending on other microbiome and host factors.72 Thus, the health consequences of menopause-related gut microbiome modification remain to be elucidated.
Menopausal Hormone Therapy and the Gut Microbiome
Rodent experiments of ovariectomy with estradiol therapy have provided proof-of-principle that gut microbiome alterations related to ovariectomy can be reversed with estrogen treatment.73–75 For example, in female and male C57BL/6 mice, sexual dimorphism of the gut microbiome (ie, lower Proteobacteria and LPS biosynthesis in females) was attenuated upon female ovariectomy, and restored with estradiol treatment.73 However, it is not yet clear whether menopausal hormone therapy reverses menopause-related changes in the gut microbiome in humans, as it does in animal models, due to a dearth of research in this area. A study of 17β-estradiol hormone therapy in women with POI (n = 20 with POI [10/10 treated/not treated], n = 10 healthy controls) reported that hormone therapy partially attenuated POI-related gut microbiome dysbiosis, in that the elevated abundance of Eggerthella in POI compared with healthy women was reversed with therapy.76 Studies of hormone therapy and the gut microbiome in naturally menopausal women are needed to determine whether hormone therapy may benefit gut health in post-menopause.
Research Gaps and Future Directions
The research to date on menopause and the gut microbiome has just begun to scratch the surface of this complex relationship. While human studies support the existence of differences in the gut microbiome by menopause status, small sample sizes in many of the studies likely hindered robust agreement in findings between studies. Additionally, no studies have examined longitudinal within-person trends in gut microbiome across the menopausal transition – this would allow for gold-standard analysis of whether changes in microbiome diversity and taxon abundance accelerate during the menopausal transition relative to a linear pattern of aging, as has been interrogated for other health characteristics in leading studies of menopause such as the SWAN.2,77,78
In addition to identifying changes in the gut microbiome during menopause, there are other gaps in knowledge regarding the menopause and gut microbiome relationship. (1) What are the mechanisms by which menopause may influence the gut microbiome? We posit that declines in estradiol and progesterone (ie, removal of metabolic substrate) would directly impact the gut microbiome, but are other indirect mechanisms also involved, such as hormonal influences on gut motility and/or immunity which affect the gut microbiome? In particular, progesterone suppresses the immune system thereby increasing susceptibility to pathogens,79–81 and it slows gastrointestinal motility82 which is an important determinant of the gut microbiome.83 (2) Does the gut microbiome influence the timing of menopause or the symptoms of menopause, through its actions in recycling estrogens or other mechanisms? For example, the gut estrobolome may provide a source of estrogens for the vaginal microbiota, which play an important role in genitourinary health and are profoundly impacted by the loss of ovarian estradiol during menopause.84 (3) Does menopausal hormone therapy impact the gut microbiome favorably, and in turn do the gut microbiota modulate the effectiveness of hormone therapy? (4) Finally, are menopause-related health sequelae mediated by the gut microbiome? Animal models suggest a causal role of the gut microbiome in potentiating disease states induced by ovariectomy, including metabolic syndrome,73 non-alcoholic fatty liver disease,85 osteoporosis,86,87 and atherosclerosis.74 For example, enhanced microbial translocation due to ovariectomy has been shown to induce bone loss in mice, and a less diverse and altered gut microbiome88,89 and higher gut permeability62 have been associated with lower bone mineral density in studies of post-menopausal women. In our own research, we have observed that menopause-related gut microbiome changes were associated with an adverse cardiometabolic risk factor profile in post-menopausal women, including lower HDL, higher waist circumference, and higher blood pressure.50 Additional research is needed to determine the contribution of the gut microbiome to menopause-related disease risk in humans.
Conclusions
In summary, the research to date on menopause, female sex hormones, and the gut microbiome suggests that menopause and/or low estrogens are associated with reduced gut microbiome diversity and estrobolome potential, and greater similarity to men in microbiome composition (Figure 2). Additionally, declines in estradiol and progesterone may lead to permeability of the gut barrier, allowing microbial translocation to occur. However, much of this is speculated based on studies with small sample sizes and heterogeneous findings. Additional research in large study populations is needed to confirm these putative effects of menopause, and to identify replicable associations of menopause with gut microbiome taxa.
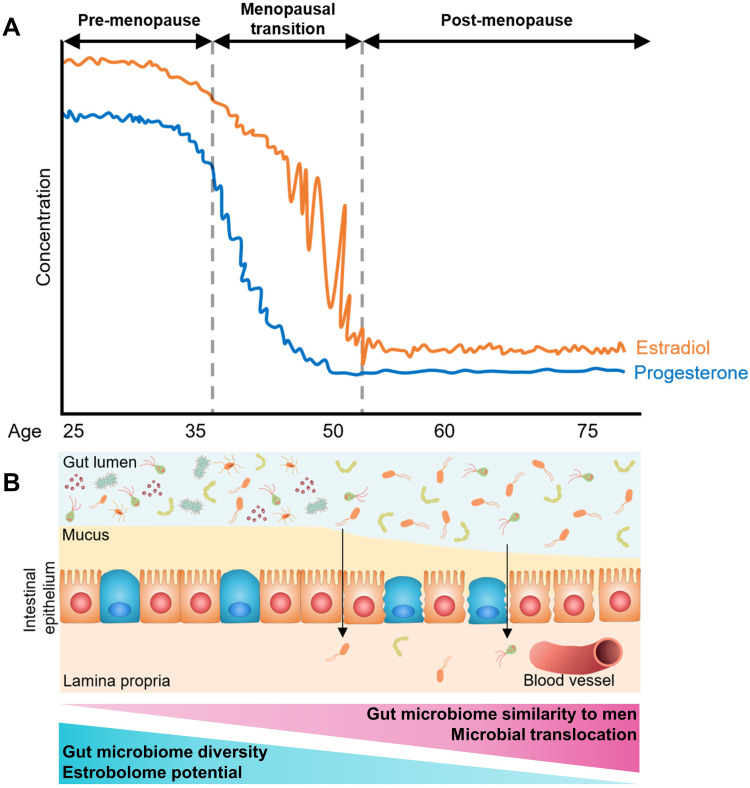
Figure 2.
Summary of putative changes in the human gut microbiome related to menopause. (A) Trajectory of estradiol and progesterone concentration during a woman’s adulthood, showing declines during the menopausal transition and low levels post-menopause. (B) Diagram of putative gut microbiome and gut epithelium changes during menopause. With declining estradiol and progesterone, diversity of the gut microbiome and estrobolome potential is reduced, and microbiome composition becomes more similar to men. Additionally, declines in estradiol and progesterone may lead to permeability of the gut barrier, allowing microbial translocation to occur.
The gut microbiome, with its capacity to deconjugate and recycle estrogens, progesterone, and other hormones, may play an important role in determining levels of sex hormones post-menopause. Given the potential to modify the gut microbiome through specific prebiotic, probiotic, or antibiotic drugs, the gut microbiome represents an unexplored new frontier in peri- and post-menopausal health. For example, could the gut microbiome be harnessed to improve menopause symptoms, effectiveness of hormone therapy, or menopause-related health outcomes? Better understanding of changes in the gut microbiome during menopause, and the function of these microbiota in symptoms and health sequelae of menopause, may open the door to this new frontier and ultimately improve the health and well-being of women in the post-reproductive years.
Acknowledgments
BAP and RCK were supported by the National Heart, Lung, and Blood Institute (BAP K01HL160146; RCK R01MD011389).
Disclosure
Dr. Santoro is a consultant with Ansh Labs and ASTELLAS/Ogeda, and receives grant support from Menogenix, Inc. outside the submitted work. All other authors declare no competing financial interests.
References
- 1.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the study of women’s health across the nation. Arch Intern Med. 2008;168(14):1568–1575. doi: 10.1001/archinte.168.14.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samargandy S, Matthews Karen A, Brooks Maria M, et al. Arterial stiffness accelerates within 1 year of the final menstrual period. Arterioscler Thromb Vasc Biol. 2020;40(4):1001–1008. doi: 10.1161/ATVBAHA.119.313622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Khoudary SR, Aggarwal B, Beckie TM, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142(25):e506–e532. doi: 10.1161/CIR.0000000000000912 [DOI] [PubMed] [Google Scholar]
- 4.Menazza S, Murphy E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res. 2016;118(6):994–1007. doi: 10.1161/CIRCRESAHA.115.305376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santoro N, Roeca C, Peters BA, Neal-Perry G. The menopause transition: signs, symptoms, and management options. J Clin Endocrinol Metab. 2020;106(1):1–5. [DOI] [PubMed] [Google Scholar]
- 6.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2009;30(4):343–375. doi: 10.1210/er.2008-0016 [DOI] [PubMed] [Google Scholar]
- 7.White Iii RA, Callister SJ, Moore RJ, Baker ES, Jansson JK. The past, present and future of microbiome analyses. Nat Protocols. 2016;11(11):2049–2053. doi: 10.1038/nprot.2016.148 [DOI] [Google Scholar]
- 8.Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, et al.; Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–1697. doi: 10.1126/science.1177486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24(4):392–400. doi: 10.1038/nm.4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi: 10.1038/s41579-020-0433-9 [DOI] [PubMed] [Google Scholar]
- 12.Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241–255. doi: 10.1038/s41579-020-00460-0 [DOI] [PubMed] [Google Scholar]
- 13.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–209. [PMC free article] [PubMed] [Google Scholar]
- 14.Knight R, Callewaert C, Marotz C, et al. The microbiome and human biology. Annu Rev Genomics Hum Genet. 2017;18:65–86. doi: 10.1146/annurev-genom-083115-022438 [DOI] [PubMed] [Google Scholar]
- 15.Mosca A, Leclerc M, Hugot JP. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol. 2016;7:455. doi: 10.3389/fmicb.2016.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart CJ, Ajami NJ, O’Brien JL, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562(7728):583–588. doi: 10.1038/s41586-018-0617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odamaki T, Kato K, Sugahara H, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korpela K, Kallio S, Salonen A, et al. Gut microbiota develop towards an adult profile in a sex-specific manner during puberty. Sci Rep. 2021;11(1):23297. doi: 10.1038/s41598-021-02375-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yurkovetskiy L, Burrows M, Khan AA, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–412. doi: 10.1016/j.immuni.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markle JGM, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521 [DOI] [PubMed] [Google Scholar]
- 22.de la Cuesta-Zuluaga J, Kelley ST, Chen Y, et al. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems. 2019;4(4):e00261–00219. doi: 10.1128/mSystems.00261-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilmanski T, Diener C, Rappaport N, et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. 2021;3(2):274–286. doi: 10.1038/s42255-021-00348-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badal VD, Vaccariello ED, Murray ER, et al. The gut microbiome, aging, and longevity: a systematic review. Nutrients. 2020;12(12):3759. doi: 10.3390/nu12123759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509(7500):357–360. doi: 10.1038/nature13178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–564. doi: 10.1126/science.aad3503 [DOI] [PubMed] [Google Scholar]
- 27.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–569. doi: 10.1126/science.aad3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha T, Vich Vila A, Garmaeva S, et al. Analysis of 1135 gut metagenomes identifies sex-specific resistome profiles. Gut Microbes. 2019;10(3):358–366. doi: 10.1080/19490976.2018.1528822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takagi T, Naito Y, Inoue R, et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J Gastroenterol. 2019;54(1):53–63. doi: 10.1007/s00535-018-1488-5 [DOI] [PubMed] [Google Scholar]
- 30.Oki K, Toyama M, Banno T, Chonan O, Benno Y, Watanabe K. Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol. 2016;16(1):284. doi: 10.1186/s12866-016-0898-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst. 2016;108(8):djw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10(4):324–335. doi: 10.1016/j.chom.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin F, Peltonen J, Laatikainen T, Pulkkinen M, Adlercreutz H. Excretion of progesteone metabolites and estriol in faeces from pregnant women during ampicillin administration. J Steroid Biochem. 1975;6(9):1339–1346. doi: 10.1016/0022-4731(75)90363-5 [DOI] [PubMed] [Google Scholar]
- 34.Colldén H, Landin A, Wallenius V, et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am J Physiol Endocrinol Metab. 2019;317(6):E1182–E1192. doi: 10.1152/ajpendo.00338.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandberg AA, Slaunwhite WR. Studies on phenolic steroids in human subjects. II. The metabolic fate and hepato-biliary-enteric circulation of C14-estrone and C14-estradiol in women. J Clin Invest. 1957;36(8):1266–1278. doi: 10.1172/JCI103524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adlercreutz H, Martin F. Biliary excretion and intestinal metabolism of progesterone and estrogens in man. J Steroid Biochem. 1980;13(2):231–244. doi: 10.1016/0022-4731(80)90196-X [DOI] [PubMed] [Google Scholar]
- 37.Adlercreutz H, Järvenpää P. Assay of estrogens in human feces. J Steroid Biochem. 1982;17(6):639–645. doi: 10.1016/0022-4731(82)90565-9 [DOI] [PubMed] [Google Scholar]
- 38.Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol. 2008;66(3):487–495. doi: 10.1111/j.1574-6941.2008.00520.x [DOI] [PubMed] [Google Scholar]
- 39.Pollet RM, D’Agostino EH, Walton WG, et al. An atlas of β-glucuronidases in the human intestinal microbiome. Structure. 2017;25(7):967–977.e965. doi: 10.1016/j.str.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ervin SM, Simpson JB, Gibbs ME, et al. Structural insights into endobiotic reactivation by human gut microbiome-encoded sulfatases. Biochemistry. 2020;59(40):3939–3950. doi: 10.1021/acs.biochem.0c00711 [DOI] [PubMed] [Google Scholar]
- 41.Ervin SM, Li H, Lim L, et al. Gut microbiome–derived β-glucuronidases are components of the estrobolome that reactivate estrogens. J Biol Chem. 2019;294(49):18586–18599. doi: 10.1074/jbc.RA119.010950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flores R, Shi J, Fuhrman B, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. doi: 10.1186/1479-5876-10-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuhrman BJ, Feigelson HS, Flores R, et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014;99(12):4632–4640. doi: 10.1210/jc.2014-2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Reau AJ, Suen G. The Ruminococci: key symbionts of the gut ecosystem. J Microbiol. 2018;56(3):199–208. doi: 10.1007/s12275-018-8024-4 [DOI] [PubMed] [Google Scholar]
- 45.Shin J-H, Park Y-H, Sim M, Kim S-A, Joung H, Shin D-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170(4):192–201. doi: 10.1016/j.resmic.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Zhuo Y, Liu Y, Chen Y, Ning Y, Yao J. Association between premature ovarian insufficiency and gut microbiota. BMC Pregnancy Childbirth. 2021;21(1):418. doi: 10.1186/s12884-021-03855-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos-Marcos JA, Rangel-Zuniga OA, Jimenez-Lucena R, et al. Influence of gender and menopausal status on gut microbiota. Maturitas. 2018;116:43–53. doi: 10.1016/j.maturitas.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 48.Mayneris-Perxachs J, Arnoriaga-Rodríguez M, Luque-Córdoba D, et al. Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: influences of obesity and menopausal status. Microbiome. 2020;8(1):136. doi: 10.1186/s40168-020-00913-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mihajlovic J, Leutner M, Hausmann B, et al. Combined hormonal contraceptives are associated with minor changes in composition and diversity in gut microbiota of healthy women. Environ Microbiol. 2021;23(6):3037–3047. doi: 10.1111/1462-2920.15517 [DOI] [PubMed] [Google Scholar]
- 50.Peters BA, Lin J, Qi Q, et al. Menopause is associated with an altered gut microbiome and estrobolome with implications for adverse cardiometabolic risk in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). mSystems. 2022;7(3). doi: 10.1128/msystems.00273-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30(1):149–173. doi: 10.1146/annurev-immunol-020711-075001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Homma H, Hoy E, Xu D-Z, Lu Q, Feinman R, Deitch EA. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol. 2005;288(3):G466–G472. doi: 10.1152/ajpgi.00036.2004 [DOI] [PubMed] [Google Scholar]
- 53.Diebel ME, Diebel LN, Manke CW, Liberati DM. Estrogen modulates intestinal mucus physiochemical properties and protects against oxidant injury. J Trauma Acute Care Surg. 2015;78(1):94–99. doi: 10.1097/TA.0000000000000499 [DOI] [PubMed] [Google Scholar]
- 54.Langen ML-V, Hotte N, Dieleman LA, Albert E, Mulder C, Madsen KL. Estrogen receptor-β signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G621–G626. doi: 10.1152/ajpgi.00274.2010 [DOI] [PubMed] [Google Scholar]
- 55.Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2009;587(Pt 13):3317–3328. doi: 10.1113/jphysiol.2009.169300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Giessen J, van der Woude CJ, Peppelenbosch MP, Fuhler GM, Direct A. Effect of sex hormones on epithelial barrier function in inflammatory bowel disease models. Cells. 2019;8(3):261. doi: 10.3390/cells8030261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Z, Bian C, Luo Z, et al. Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci Rep. 2019;9(1):8367. doi: 10.1038/s41598-019-44448-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collins FL, Rios-Arce ND, Atkinson S, et al. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol Rep. 2017;5(9):e13263. doi: 10.14814/phy2.13263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Z, Powell AM, Ramakrishnan V, Eckard A, Wagner C, Jiang W. Elevated systemic microbial translocation in pregnant HIV-infected women compared to HIV-uninfected women, and its inverse correlations with plasma progesterone levels. J Reprod Immunol. 2018;127:16–18. doi: 10.1016/j.jri.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh M, Rodriguez-Garcia M, Wira CR. The immune system in menopause: pros and cons of hormone therapy. J Steroid Biochem Mol Biol. 2014;142:171–175. doi: 10.1016/j.jsbmb.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grishina I, Fenton A, Sankaran-Walters S. Gender differences, aging and hormonal status in mucosal injury and repair. Aging Dis. 2014;5(2):160–169. doi: 10.14336/AD.2014.0500160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shieh A, Epeldegui M, Karlamangla AS, Greendale GA. Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight. 2020;5(2). doi: 10.1172/jci.insight.134092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peters BA, Xue X, Sheira LA, et al. Menopause is associated with immune activation in women with HIV. J Infect Dis. 2021;225(2):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao H, Chen J, Li X, Sun Q, Qin P, Wang Q. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Lett. 2019;593(18):2655–2664. doi: 10.1002/1873-3468.13527 [DOI] [PubMed] [Google Scholar]
- 65.Peters BA, Xue X, Wang Z, et al. Menopausal status and observed differences in the gut microbiome in women with and without HIV infection. Menopause. 2021;28(5):491–501. doi: 10.1097/GME.0000000000001730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jha AR, Davenport ER, Gautam Y, et al. Gut microbiome transition across a lifestyle gradient in Himalaya. PLoS Biol. 2018;16(11):e2005396. doi: 10.1371/journal.pbio.2005396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaplan RC, Wang Z, Usyk M, et al. Gut microbiome composition in the Hispanic Community Health Study/Study of Latinos is shaped by geographic relocation, environmental factors, and obesity. Genome Biol. 2019;20(1):219. doi: 10.1186/s13059-019-1831-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao D, Guallar E, Ouyang P, et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol. 2018;71(22):2555–2566. doi: 10.1016/j.jacc.2018.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–591. doi: 10.1038/nrendo.2015.128 [DOI] [PubMed] [Google Scholar]
- 70.Pinart M, Dötsch A, Schlicht K, et al. Gut microbiome composition in obese and non-obese persons: a systematic review and meta-analysis. Nutrients. 2021;14(1):12. doi: 10.3390/nu14010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tett A, Pasolli E, Masetti G, Ercolini D, Segata N. Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol. 2021;19(9):585–599. doi: 10.1038/s41579-021-00559-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wexler AG, Goodman AL. An insider’s perspective: bacteroides as a window into the microbiome. Nature Microbiol. 2017;2(5):17026. doi: 10.1038/nmicrobiol.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaliannan K, Robertson RC, Murphy K, et al. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome. 2018;6(1):205. doi: 10.1186/s40168-018-0587-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meng Q, Ma M, Zhang W, et al. The gut microbiota during the progression of atherosclerosis in the perimenopausal period shows specific compositional changes and significant correlations with circulating lipid metabolites. Gut Microbes. 2021;13(1):1–27. doi: 10.1080/19490976.2021.1880220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeong SY, Kang S, Hua CS, Ting Z, Park S. Synbiotic effects of β-glucans from cauliflower mushroom and Lactobacillus fermentum on metabolic changes and gut microbiome in estrogen-deficient rats. Genes Nutr. 2017;12:31. doi: 10.1186/s12263-017-0585-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang L, Fei H, Tong J, et al. Hormone replacement therapy reverses gut microbiome and serum metabolome alterations in premature ovarian insufficiency. Front Endocrinol. 2021;12:794496. doi: 10.3389/fendo.2021.794496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res. 2012;27(1):111–118. doi: 10.1002/jbmr.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–2373. doi: 10.1016/j.jacc.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hall OJ, Klein SL. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol. 2017;10(5):1097–1107. doi: 10.1038/mi.2017.35 [DOI] [PubMed] [Google Scholar]
- 80.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62(3):263–271. doi: 10.1016/j.yhbeh.2012.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao Y, Li H, Ding J, Xia Y, Wang L, Zhang G. Progesterone impairs antigen-non-specific immune protection by CD8 T memory cells via interferon-γ gene hypermethylation. PLoS Pathog. 2017;13(11):e1006736. doi: 10.1371/journal.ppat.1006736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coquoz A, Regli D, Stute P. Impact of progesterone on the gastrointestinal tract: a comprehensive literature review. Climacteric. 2022;25:1–25. [DOI] [PubMed] [Google Scholar]
- 83.Asnicar F, Leeming ER, Dimidi E, et al. Blue poo: impact of gut transit time on the gut microbiome using a novel marker. Gut. 2021;70(9):1665. doi: 10.1136/gutjnl-2020-323877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Łaniewski P, Herbst-Kralovetz MM. Connecting microbiome and menopause for healthy ageing. Nature Microbiol. 2022;7(3):354–358. doi: 10.1038/s41564-022-01071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu L, Fu Q, Li T, et al. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. PLoS One. 2022;17(2):e0262855. doi: 10.1371/journal.pone.0262855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuan Y, Yang J, Zhuge A, Li L, Ni S. Gut microbiota modulates osteoclast glutathione synthesis and mitochondrial biogenesis in mice subjected to ovariectomy. Cell Prolif. 2022;55(3):e13194. doi: 10.1111/cpr.13194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu M, Pal S, Paterson CW, et al. Ovariectomy induces bone loss via microbial-dependent trafficking of intestinal TNF+ T cells and Th17 cells. J Clin Invest. 2021;131(4). doi: 10.1172/JCI143137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rettedal EA, Ilesanmi-Oyelere BL, Roy NC, Coad J, Kruger MC. The gut microbiome is altered in postmenopausal women with osteoporosis and osteopenia. JBMR Plus. 2021;5(3):e10452. doi: 10.1002/jbm4.10452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He J, Xu S, Zhang B, et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging. 2020;12(9):8583–8604. doi: 10.18632/aging.103168 [DOI] [PMC free article] [PubMed] [Google Scholar]