Figure 2.
Myristoylated BASP1 is present in the nucleus and is recruited to the promoter regions of WT1 target genes
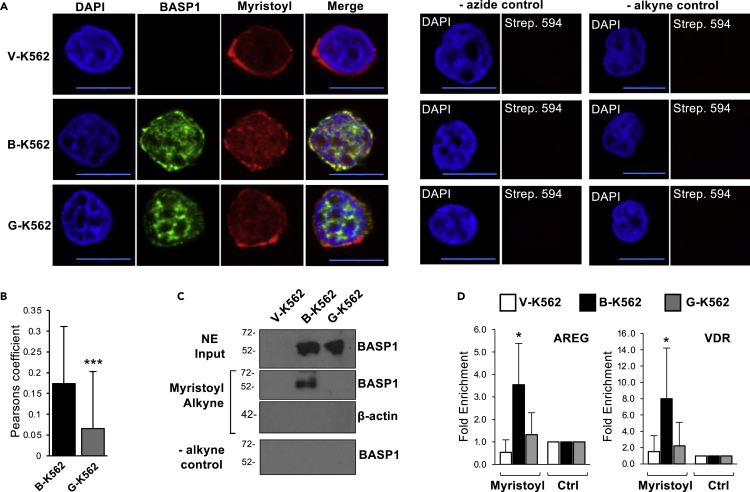
(A) The indicated cell lines were incubated in lipid-free media in the presence of 10 μg/mL myristic acid alkyne for 20 h. Nuclei were prepared and incubated with azide PEG3-biotin, then the click reaction was initiated using 2mM CuBF4. Immunohistochemistry was then performed with streptavidin-linked antibodies (Myristoyl) and BASP1 antibodies. Scale bar (blue) is 10μm. Control assays were performed that lacked azide PEG3-biotin (-azide control) or myristic acid alkyne (-alykyne control) and are shown at right.
(B) Quantification of the colocalization of BASP1 and Myristoyl in B-K562 and G-K562 nuclei was analyzed using Pearson’s correlation coefficient. ∗∗∗ = p < 0.005 following students t-test comparing Pearson’s values from BK and GK nuclei over three independent experiments (n = 67).
(C) V-K562, B-K562, and G-K562 cells were incubated with ethanol (-alkyne control) or 10 μg/mL alkyne-Myristic acid as above and subjected to a click chemistry reaction. Nuclear extracts were prepared and precipitation was performed with streptavidin beads. Immunoblotting was then performed with either BASP1 antibodies or control β-actin antibodies. Molecular weight marker in kDa shown to left of each blot. Gels are representative of three independent experiments.
(D) The cell line derivatives were treated in part A and following the click chemistry reaction, ChIP was performed with either streptavidin-linked beads (Myristoyl) or control beads (Ctrl). Data are shown as fold enrichment of Myristoyl at the AREG and VDR promoters in V-K562, B-K562, and G-K562 cells compared to the alkyne-free control precipitation. Error bars are SDM of ten independent experiments with ∗ = p < 0.05 by student’s t test comparing B-K562 or G-K562 with control cell line V-K562.