Abstract
BACKGROUND
The authors present two cases of paradoxical ventriculomegaly after lumboperitoneal (LP) shunting in patients with slit ventricle syndrome (SVS).
OBSERVATIONS
After placement of an LP shunt, both patients rapidly developed radiographic and clinically symptomatic ventricular enlargement. The then generous ventricular corridors allowed both patients to be treated by endoscopic third ventriculostomy (ETV) with concurrent removal of their LP shunt. The patients then underwent staged increases in their shunt resistance to the maximum setting and remain asymptomatic.
LESSONS
The authors suggest that this paradoxical ventriculomegaly may have resulted from a pressure gradient between the shunt systems in the intra- and extraventricular spaces due to a noncommunicating etiology of their hydrocephalus. ETV may successfully exploit this newfound obstructive hydrocephalus and provide resolution of the radiographic and clinical hydrocephalus through allowing for improved communication between the cranial and lumbar cerebrospinal fluid spaces in SVS.
Keywords: slit ventricle syndrome, endoscopic third ventriculostomy, ventriculoperitoneal shunt, lumboperitoneal shunt, pediatric neurosurgery, hydrocephalus
ABBREVIATIONS : CSF = cerebrospinal fluid, CT = computed tomography, EVD = external ventricular drain, ETV = endoscopic third ventriculostomy, IIH = intracranial hypertension, LP = lumboperitoneal, SVS = slit ventricle syndrome, VP = ventriculoperitoneal shunting
Slit ventricle syndrome (SVS) is a challenging clinical presentation of small ventricles with abnormal morphology that remain small despite elevations in intracranial pressure that poses challenges for ventricular shunt maintenance and revision.1 The pathogenesis of SVS is controversial and multifactorial. Common theories include decreased compliance of the ependymal walls, change in brain turgor, chronic venous congestion, cranial vault disproportion, and ventricular exclusion.2 Development of SVS may be impacted by shunt placement during infancy, although the condition may be acquired at any age after any amount of time.3
There is a lack of consensus regarding optimal treatment of shunt failure in SVS. Placement of a ventricular catheter may require image guidance due to small ventricular chambers, while endoscopic third ventriculostomy (ETV) is challenging due to the narrow working ventricular corridor. According to a 2017 survey of pediatric neurosurgeons, the most commonly preferred treatments of SVS include ventriculoperitoneal (VP) shunting, cranial expansion, antisiphon device placement, and ETV.4 Lumboperitoneal (LP) shunts, cisternal shunts,5 additional valve or shunt resistance, and abdominal binders have also been described.4,6,7
We present two cases of chronically shunted pediatric patients with SVS who were transitioned to LP shunt after VP shunt revisions failed to provide symptomatic relief. Postoperatively, both patients experienced rapid clinical decline with symptoms of intracranial hypertension and ventriculomegaly. Both patients then underwent LP shunt removal with concurrent ETV, leading to rapid clinical and radiographic improvement and eventual shunt freedom.
Illustrative Cases
Case 1
A 15-year-old male with a history of intraventricular hemorrhage of prematurity and posthemorrhagic hydrocephalus and numerous VP shunt revisions during infancy presented with several months of severe positional headaches, vision changes, and dizziness after 12 years of stability. Imaging revealed slit ventricles (Fig. 1A and B). Over the following 2 years, he underwent numerous shunt interventions including proximal revisions, placement of a contralateral shunt, and insertion of programmable valves and multiple adjustments of his shunt resistances. Unfortunately, none of the above interventions provided durable relief of his positional headaches.
FIG. 1.
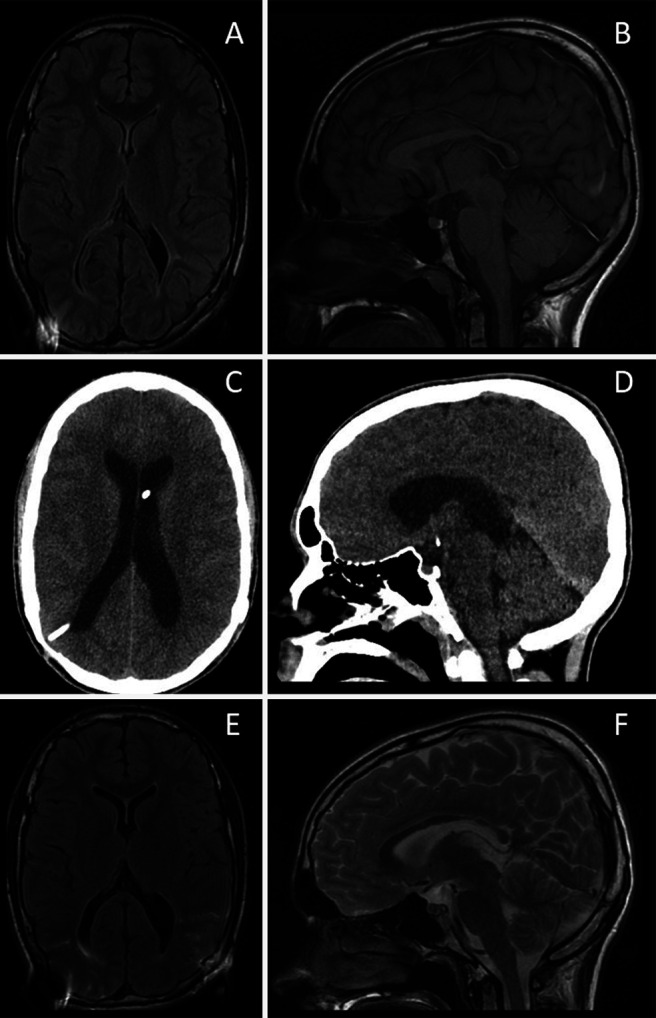
Radiographic findings in case 1. A and B: Brain MRI FLAIR sequence during symptomatic hydrocephalus showing SVS. C and D: Noncontrast head CT showing ventriculomegaly after LP shunt placement and lack of an acquired Chiari malformation. E and F: Brain MRI FLAIR sequence showing reduction in ventricular size after LP shunt removal and endoscopic third ventriculostomy. FLAIR = fluid-attenuated inversion recovery; MRI = magnetic resonance imaging.
The patient eventually underwent placement of a nonprogrammable slit valve LP shunt (Integra Neurosciences Spetzler). On the first postoperative day he became increasingly lethargic and developed new headaches while supine. Computed tomography (CT) scan of the head demonstrated new ventriculomegaly (Fig. 1C and D). Given his radiographic findings and worse clinical status, the patient was brought to surgery to attempt endoscopic third ventriculostomy and remove his new LP shunt. The ETV was feasible only because of his enlarged ventricles. His opening pressure was approximately 20 cm water (H2O) his VP shunt was noted to be functioning with brisk proximal flow and rapid distal runoff and was thus left in place without revision. His ventricles then normalized without returning to slit morphology (Fig. 1E and F). The patient underwent staged valve adjustments until his VP shunt valve reached the maximum resistance of 200 mm H2O. This was done both to ensure high enough pressure to protect patency of the ventriculostomy and to attempt shunt freedom in a controlled fashion, which he continues to tolerate well 24 months after surgery.
Case 2
A 4-year-old male with slit ventricles presented with symptoms characteristic of prior shunt failures (Fig. 2A and B). He had a history of obstructive hydrocephalus secondary to aqueductal stenosis and a large left-sided porencephalic cyst status treated by endoscopic third ventriculostomy at 6 months of age that failed, followed by a VP shunt placement and two subsequent revisions in infancy. He thus underwent a proximal shunt revision but did not improve despite the functioning VP shunt. A slit valve LP shunt was then placed to augment cerebrospinal fluid (CSF) diversion. He initially experienced significant symptomatic improvement. However, on the second postoperative day he developed worsening headache, nausea, and lethargy and was found to have new ventriculomegaly (Fig. 2C and D). This prompted urgent return to the operating room for removal of the new LP shunt with simultaneous refenestration of the healed ETV and replacement of his VP shunt valve with a programmable Codman Certas Plus valve (Integra LifeSciences). The decision was made to attempt ETV given the unique opportunity of his newly enlarged ventricular corridor. His intracranial pressure was approximately 20 cm H2O and his recently revised shunt was functioning well. The patient subsequently experienced marked symptomatic improvement. Over the course of the following week, his shunt valve was gradually adjusted to the highest resistance, equivalent to 40 cm H2O (Fig. 2E and F), to maintain patency of the ventriculostomy. The patient has tolerated this “off” valve setting well. He required a revision of his ETV at 4 months and 12 months postoperatively due to headaches and a CINE flow study demonstrating closure of the ventriculostomy, which has provided good relief. He has not required any shunt adjustments and remains in the “off” position 12 months after removal of the LP shunt.
FIG. 2.
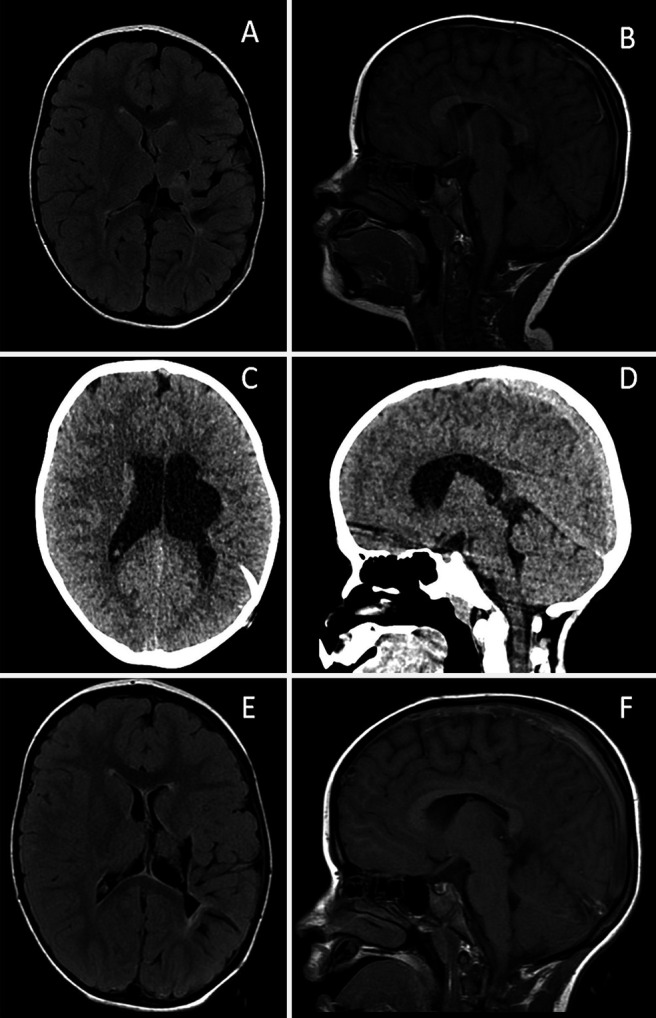
Radiographic findings in case 2. A and B: Brain MRI FLAIR sequence during symptomatic hydrocephalus showing SVS. C and D: Noncontrast head CT showing ventriculomegaly after LP shunt placement and lack of an acquired Chiari malformation. E and F: Brain MRI FLAIR sequence showing reduction in ventricular size after LP shunt removal and endoscopic third ventriculostomy. FLAIR = fluid-attenuated inversion recovery; MRI = magnetic resonance imaging.
Discussion
Observations
We present two cases in which chronically shunted pediatric patients rapidly developed symptomatic ventriculomegaly after placement of an LP shunt intended to augment CSF diversion. ETV and simultaneous removal of the LP shunt was successful in both cases in resolving their hydrocephalus and removing the need for further shunting.
SVS is a complex consequence of long-term ventricular shunting in which patients may experience intracranial hypertension without changes in ventricular size despite shunt revisions or modifications of a programmable valve.5 For patients with communicating hydrocephalus and small ventricles, an LP shunt has been shown to be a safe and occasionally more reliable alternative to a VP shunt. To our knowledge, we provide the first description of rapid onset ventricular enlargement after placement of an LP shunt.
There are certain contraindications to an LP shunt, which can be best summarized by the consensus guidelines for lumbar puncture: presence of space-occupying lesions with mass effect, abnormal intracranial pressure due to increased CSF pressure, and Arnold-Chiari malformation could potentially increase the risk of herniation during lumbar shunting.8 An acquired Chiari malformation over time has also been described.9,10
We could not find similar cases in the literature describing new ventriculomegaly after LP shunt placement. There is a case published from Australia describing tonsillar herniation after lumbar puncture in a patient with intracranial hypertension (IIH). A 30-year-old female with known IIH who had previously undergone multiple uneventful lumbar punctures experienced rapid neurological decline including extensor posturing and anisocoria 7 hours after lumbar puncture requiring external ventricular drain (EVD) and bifrontal craniectomy for decompression. Her imaging demonstrated severe diffuse cerebral edema and clear cerebellar tonsillar descent through the foramen magnum.11 In fact, development of Chiari malformation is often cited as surgeons’ hesitation to place LP shunts in the first place. Chumas et al.10 published a series in 1993 of patients treated with LP shunts between 1974 and 1991 and The Hospital for Sick Children, Toronto. They found that 6 of 143 patients treated with LP shunts developed symptomatic tonsillar herniation requiring intervention including one death. They additionally state that a majority of patients who were asymptomatic still had radiographic evidence of hindbrain herniation. In fact, multiple case reports have demonstrated an acquired Chiari I malformation after LP shunt.12,13 The significant difference from our case is the clear development of tonsillar descent causing brainstem compression and obstructive hydrocephalus, which were absent in both of our cases.
CSF is assumed to be freely communicating throughout the head and spine in a normal individual. For example, a lumbar puncture is assumed to be diagnostic for cerebral meningitis, and the entire CSF space is considered treated when injecting an Ommaya reservoir with chemotherapy. Marupudi et al.6 published a large series supporting the use of LP shunting for pediatric patients with slit ventricles. The authors retrospectively reviewed 143 patients who successfully underwent LP shunting for either IIH or slit ventricles. In this series, the authors followed a careful protocol for converting patients from VP shunt to LP shunt. involving externalization of VP shunt to EVD while simultaneously placing lumbar drain. The EVD was slowly weaned over several days and then a CT ventriculogram was performed to rule out any obstruction. If there was obstruction, ETV was often performed and then both drains were clamped. For those who failed clamp trial or were not ETV candidates, EVD was clamped while the lumbar drain was open to ensure that the patient tolerated purely lumbar drainage. A horizontal-vertical valve was used to reduce overdrainage when upright. Additionally, the authors found that none of 31 patients with known cerebellar ectopia or Chiari malformation progressed on serial imaging, although patients with symptomatic Chiari malformations were never included because they were not deemed candidates for LP shunt.6 Unfortunately, the authors do not report on the patients who were excluded for not tolerating the lumbar drain trial if there were any.
In the case of our two patients, the placement of LP shunts resulted in rapid development of ventriculomegaly. In both cases patients had functional VP shunts in place confirmed at surgery. The subsequent response to ETV suggests both patients had a noncommunicating etiology for the hydrocephalus. The paradoxical effect of ventricular enlargement may be explained by the creation of a pressure gradient between the ventricles within the brain and the CSF space outside of the brain caused by the LP shunt drainage. The negative pressure in the extraventricular space generated by the LP shunt led to inflation of the ventricles given the lack of communication between the two systems. The mechanism might be similar to the inflation of pulmonary alveoli from atmospheric pressure that exceeds the negative pressure generated within the thoracic space by the diaphragm on inhalation. Paradoxical ventriculomegaly has been described after a fourth ventricle shunt in an adolescent with SVS and a trapped fourth ventricle suggesting noncommunicating subarachnoid spaces in subpopulations with SVS.14 Interestingly, the rapid ventricular expansion in these two cases would seem to argue against decreased ependymal compliance as a cause of their individual SVS.
Although we would not recommend purposefully inducing acutely symptomatic ventriculomegaly by placement of an LP shunt, the ventricular enlargement did provide the opportunity to perform an endoscopic third ventriculostomy through widened ventricular corridors that had been considered too small for passage of our endoscope at prior shunt revisions. ETV was a pragmatic solution in both cases in which LP shunting had demonstrated convincing noncommunicating hydrocephalus. These cases illustrate the risk of LP shunt in cases of noncommunicating hydrocephalus even when functional VP shunt systems are in place.
Lessons
Here we describe unexpected ventriculomegaly and symptomatic hydrocephalus following placement of LP shunts for SVS in two patients. Both were discovered to have functioning VP shunts in place. We suggest that this paradoxical ventriculomegaly may have resulted from a pressure gradient between the shunt systems in the intra- and extraventricular spaces due to a noncommunicating etiology of their hydrocephalus. This phenomenon of paradoxical ventriculomegaly has not been described in prior literature as a potential consequence of LP shunting. The etiology of the hydrocephalus should be carefully considered prior to performing an LP shunt, even in cases of SVS and with VP shunts in place. ETV provided resolution of both the hydrocephalus and SVS by improving communication between subarachnoid spaces, even so far as freeing both patients from the need for a shunt. We hope that these two cases further contribute to the understanding of CSF flow dynamics in patients with multiple shunts and complex hydrocephalus to provide better treatment pathways for these challenging conditions.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Gonda, Plonsker, Wali. Acquisition of data: Gonda, Gilbert, Al Jammal, Wali. Analysis and interpretation of data: Gonda, Barnett, Wali. Drafting the article: Gonda, Gilbert, Barnett, Al Jammal, Wali, Gupta. Critically revising the article: Gonda, Gilbert, Plonsker, Barnett, Wali, Gupta. Reviewed submitted version of manuscript: Gonda, Gilbert, Plonsker, Gupta. Approved the final version of the manuscript on behalf of all authors: Gonda. Administrative/technical/material support: Barnett. Study supervision: Gonda.
References
- 1. Major O, Fedorcsák I, Sipos L, et al. Slit-ventricle syndrome in shunt operated children. Acta Neurochir (Wien) 1994;127(1-2):69–72. doi: 10.1007/BF01808550. [DOI] [PubMed] [Google Scholar]
- 2. Ros B, Iglesias S, Martín Á, Carrasco A, Ibáñez G, Arráez MA. Shunt overdrainage syndrome: review of the literature. Neurosurg Rev. 2018;41(4):969–981. doi: 10.1007/s10143-017-0849-5. [DOI] [PubMed] [Google Scholar]
- 3. Baskin JJ, Manwaring KH, Rekate HL. Ventricular shunt removal: the ultimate treatment of the slit ventricle syndrome. J Neurosurg. 1998;88(3):478–484. doi: 10.3171/jns.1998.88.3.0478. [DOI] [PubMed] [Google Scholar]
- 4. Kraemer MR, Sandoval-Garcia C, Bragg T, Iskandar BJ. Shunt-dependent hydrocephalus: management style among members of the American Society of Pediatric Neurosurgeons. J Neurosurg Pediatr. 2017;20(3):216–224. doi: 10.3171/2017.2.PEDS16265. [DOI] [PubMed] [Google Scholar]
- 5. Rekate HL. The slit ventricle syndrome: advances based on technology and understanding. Pediatr Neurosurg. 2004;40(6):259–263. doi: 10.1159/000083737. [DOI] [PubMed] [Google Scholar]
- 6. Marupudi NI, Harris C, Pavri T, et al. The role of lumboperitoneal shunts in managing chronic hydrocephalus with slit ventricles. J Neurosurg Pediatr. 2018;22(6):632–637. doi: 10.3171/2018.6.PEDS17642. [DOI] [PubMed] [Google Scholar]
- 7. Le H, Yamini B, Frim DM. Lumboperitoneal shunting as a treatment for slit ventricle syndrome. Pediatr Neurosurg. 2002;36(4):178–182. doi: 10.1159/000056054. [DOI] [PubMed] [Google Scholar]
- 8. Engelborghs S, Niemantsverdriet E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement (Amst) 2017;8:111–126. doi: 10.1016/j.dadm.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duthel R, Nuti C, Motuo-Fotso MJ, Beauchesne P, Brunon J. Complications of lumboperitoneal shunts. A retrospective study of a series of 195 patients (214 procedures). Article in French. Neurochirurgie. 1996;42(2):83–90. [PubMed] [Google Scholar]
- 10. Chumas PD, Armstrong DC, Drake JM, et al. Tonsillar herniation: the rule rather than the exception after lumboperitoneal shunting in the pediatric population. J Neurosurg. 1993;78(4):568–573. doi: 10.3171/jns.1993.78.4.0568. [DOI] [PubMed] [Google Scholar]
- 11. Borire AA, Hughes AR, Lueck CJ. Tonsillar Herniation After Lumbar Puncture in Idiopathic Intracranial Hypertension. J Neuroophthalmol. 2015;35(3):293–295. doi: 10.1097/WNO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 12. Hentati A, Badri M, Bahri K, Zammel I. Acquired Chiari I malformation due to lumboperitoneal shunt: a case report and review of literature. Surg Neurol Int. 2019;10:78. doi: 10.25259/SNI-234-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riffaud L, Moughty C, Henaux PL, Haegelen C, Morandi X. Acquired Chiari I malformation and syringomyelia after valveless lumboperitoneal shunt in infancy. Pediatr Neurosurg. 2008;44(3):229–233. doi: 10.1159/000121381. [DOI] [PubMed] [Google Scholar]
- 14. Marianayagam NJ, Shalom NB, Yassin S, Michowiz S, Harnof S, Rajz G. Paradoxical ventriculomegaly due to low-pressure hydrocephalus, a rare complication of the treatment of a trapped fourth ventricle: case report. J Clin Neurosci. 2017;39:101–103. doi: 10.1016/j.jocn.2017.01.017. [DOI] [PubMed] [Google Scholar]


