Abstract
Background
The prevalence of post-COVID conditions (PCC) and associated physical, psychological, and cognitive symptoms was assessed among Quebec healthcare workers (HCWs) with coronavirus disease 2019 (COVID-19).
Methods
This case-control study compared 6061 symptomatic HCWs with polymerase chain reaction–confirmed COVID-19 between July 2020 and May 2021 with a random sample of 4390 symptomatic HCWs who were test-negative controls. The prevalence of physical symptoms lasting ≥4 weeks (PCC4w) or ≥12 weeks (PCC12w) was estimated among hospitalized and nonhospitalized cases. In multivariate models, sociodemographic and clinical characteristics, as well as vaccine history, were evaluated as potential risk factors. Prevalence ratios compared 4 aspects of self-reported cognitive dysfunction among PCC cases to controls, adjusting for psychological distress and fatigue.
Results
PCC4w and PCC12w prevalences of 46% (2746/5943) and 40% (653/1746), respectively, were observed among nonhospitalized cases and 76% (90/118) and 68% (27/37), respectively, among hospitalized cases. Hospitalization, female sex, and age were associated with higher PCC risk. A substantial proportion of nonhospitalized PCC4w cases often or very often reported cognitive dysfunction, including concentration (33%) or organizing (23%) difficulties, forgetfulness (20%), and loss of necessary items (10%). All 4 aspects of cognitive dysfunction were associated with PCC4w symptoms, psychological distress, and fatigue.
Conclusions
PCC may be a frequent sequela of ambulatory COVID-19 in working-age adults, with important effects on cognition. With so many HCWs infected, the implications for quality healthcare delivery could be profound if cognitive dysfunction and other severe PCC symptoms persist in a professionally disabling way. Further evaluation of PCC prevalence and prognosis is warranted.
Keywords: cognitive dysfunction, healthcare workers, post-COVID conditions, SARS-CoV-2
Among 6061 healthcare workers in Quebec, prevalence of ≥12-week post-COVID conditions was 40% and 68% among nonhospitalized and hospitalized cases, respectively. Over 30% of nonhospitalized cases reported cognitive dysfunction, which was associated with persistent physical symptoms, psychological distress, and fatigue.
The coronavirus disease 2019 (COVID-19) pandemic qualifies as the most disruptive global health crisis in recent history. The persistence of symptoms long after the acute phase of the disease, as reported in a substantial proportion of cases, would add to and extend the already serious burden of this pandemic.
The Centers for Disease Control and Prevention (CDC) has defined post-COVID conditions (PCC) as a wide range of physical and mental health problems persisting ≥4 weeks after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1]. In October 2021, to improve specificity of this heterogeneous condition and to better represent its potentially prolonged effects, the World Health Organization (WHO) defined PCC as the persistence or relapsing of symptoms at least 12 weeks after the onset of COVID-19 without alternative diagnosis [2].
The prevalence, risk factors, and duration of PCC are still poorly understood. Systematic reviews have reported persistent symptoms among 61% (4–12 weeks), 53% (>12 weeks), or 73% (>60 days) of COVID-19 patients [3, 4]. However, hospitalized patients were overrepresented and the reviewed evidence had low certainty due to methodological limits as self-selection bias. Robust and valid estimates of the PCC burden are needed, particularly for nonhospitalized COVID-19 cases who comprise the vast majority of SARS-CoV-2 illness.
While PCC includes a large array of symptoms, neurocognitive symptoms are among the most frequent and debilitating [3, 5]. Large-scale epidemiological quantification of memory, concentration, and executive dysfunction in PCC cases is still lacking. In particular, the contribution of PCC to cognitive dysfunctions needs to take into account the potential separate contributions of fatigue and psychological distress, which are known to have deleterious effects on cognitive abilities [6, 7] and were highly prevalent among healthcare workers (HCWs) in the context of the additional enormous workload caused by the pandemic.
During the second and third waves of the COVID-19 pandemic in Quebec, Canada, a large survey was conducted to evaluate workplace exposure, infection risk, and prevention measures among SARS-CoV-2 infected (cases) and noninfected (controls) HCWs, but data were also collected on the persistence of COVID-19 symptoms at the time of completion of the questionnaire [8]. In the substudy reported here we estimated (1) the prevalence and risk factors for persistent COVID-19 physical symptoms 5–28 weeks after acute illness onset among a cohort of predominantly nonhospitalized HCWs, and (2) the contribution of PCC, fatigue, and psychological distress on persistent neurocognitive symptoms, comparing PCC cases to noninfected controls.
METHODS
Study Design and Population
A survey-based retrospective cohort study was conducted. It included HCWs of the province of Quebec tested due to COVID-19–compatible symptoms between 12 July 2020 and 29 May 2021 whose polymerase chain reaction (PCR) test was positive for SARS-CoV-2. Participants needed to be aged ≥18 years, speak English or French, have worked during the 14 days before their illness, and have completed the survey at least 5 weeks after illness onset or testing. To evaluate cognitive and psychological manifestations, a case-control design was used. Non–COVID-19 controls, recruited only in a second phase of the survey, were a random sample of HCWs tested for COVID-19–compatible symptoms between 14 November 2020 and 29 May 2021, but who were PCR negative and not previously infected.
Data Collection and Main Variables
Contact information on SARS-CoV-2–infected HCWs and controls was obtained from the provincial COVID-19 and SARS-CoV-2 laboratory databases, which together contain all confirmed cases reported to public health and all PCR-tested individuals since the beginning of the pandemic.
HCWs were called (85% of all test-positive cases and 95% of all randomly selected controls due to research team logistic constrains) between 3 December 2020 and 31 July 2021. Those reached were invited to participate in a survey aimed to identify workplace prevention and control measures of COVID-19. If eligibility criteria were met, consenting participants were asked to complete a standardized 30-minute online (97%) or phone (3%) questionnaire collecting sociodemographic data, employment characteristics, workplace exposures and prevention measures, clinical data, and COVID-19 vaccination status (Supplementary Material). HCWs were offered vaccination in Quebec beginning 14 December 2020.
Clinical questions to all SARS-CoV-2–infected HCWs included symptom onset and sick leave dates, hospitalization ≥24 hours due to COVID-19, and number of weeks until full recovery. Among cases not completely recovered at the time of the survey, information was sought about the presence and severity (mild, moderate, or severe) of 15 physical symptoms at the time of the survey, including fatigue, fever, shortness of breath, cough, wheezing, chest pain, headache, difficulty walking, loss of smell, loss of taste, joint or muscle pain, abdominal pain, diarrhea, sore throat, and runny nose. Persistent physical symptoms were not assessed among test-negative controls.
Self-reported appraisal of 4 aspects of cognitive dysfunction present at the time of the survey were queried in all participants: (1) difficulty concentrating or maintaining attention; (2) difficulty organizing oneself; (3) forgetting things; and (4) losing necessary items. Answers given on a 5-level Likert scale (never, rarely, sometimes, often, or very often), were considered positive if the problem occurred often or very often, due to low numbers of some categories. Psychological distress, which is also associated with cognitive dysfunction and/or PCC, was assessed at the moment of the survey with the validated Kessler Psychological Distress Scale, or K6 (0–24). This scale evaluates the frequency, during the preceding month, of feeling nervous, hopeless, restless, depressed, worthless, and that everything was an effort. A score of 7–12 indicates high psychological distress and of >12 a very high level of psychological distress [9].
Post-COVID Condition Definition
PCC was defined as the persistence of any of the 15 investigated physical symptoms following an acute COVID-19 disease either ≥4 weeks (PCC4w) or ≥12 weeks (PCC12w), similar to the definitions used by the CDC and WHO, respectively [1, 2]. The duration of symptoms was estimated considering the dates of survey completeness and illness onset. For example, the prevalence of PCC4w cases was calculated as the number of HCWs with persistent symptoms at the time of the survey among those who filled the survey >4 weeks after illness onset. This prevalence was similarly calculated for PCC4w and PCC12w with ≥1 severe symptom.
To estimate temporal patterns, the prevalence of symptoms was calculated among participants answering the survey at each interval from their illness onset (5–8, 9–12, 13–16, 17–20, 21–24, and 25–28 weeks).
To evaluate the contribution of COVID-19 to the reported persistent physical, cognitive, and psychological symptoms, PCC cases and prevalence were defined based on physical symptoms.
Statistical Analysis
Prevalence and binomial 95% confidence intervals (CIs) were estimated. The 4-weekly prevalence trend over time for each symptom was assessed using a Cochran-Armitage test. The correlation between symptoms was explored using the Spearman correlation coefficient.
To estimate prevalence ratios (PRs) measuring the total association between PCC, sociodemographic characteristics, vaccination status, and hospitalization with PCC, 3 log-binomial models were built: (1) evaluating only sociodemographic characteristics; (2) evaluating vaccination status adjusted for sociodemographic characteristics; and (3) evaluating hospitalization adjusted for sociodemographic characteristics and vaccination status [10].
The association between cognitive dysfunction and PCC, psychological distress, and fatigue was assessed with PRs using robust Poisson comparing PCC4w cases with SARS-CoV-2 test-negative controls, adjusting for sociodemographic characteristics.
Analysis were performed using SAS version 9.4 software (SAS Institute).
Patient Consent Statement
All participants gave verbal consent during the phone contact and electronic consent at the beginning of the survey. This study was approved by the research ethics committee of the Centre hospitalier universitaire de Québec–Université Laval.
RESULTS
Population
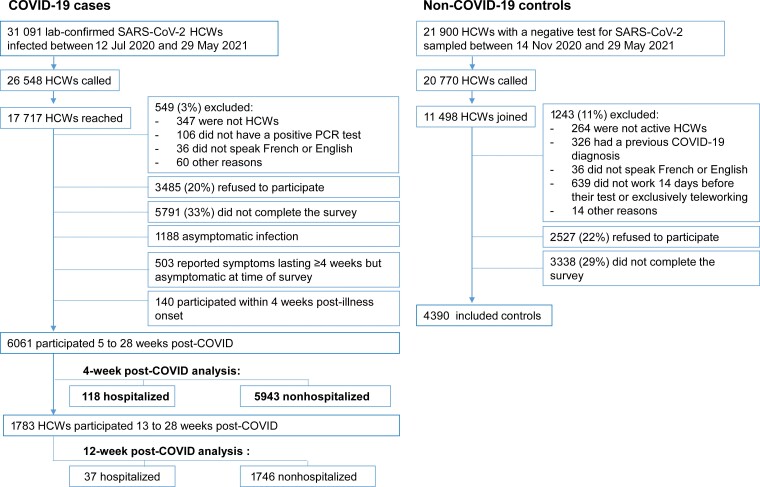
Of the 31 091 HCWs infected with SARS-CoV-2 in Quebec during the study period, 26 548 were called and 17 717 were reached (Figure 1). Inclusion criteria were not met by 549 (3%), 3485 (20%) refused to participate, and 5791 (33%) did not complete the questionnaire (missing data on clinical and/or demographic variables). Among those who answered the questionnaire, 1831 were excluded: 140 answered the survey within 4 weeks from illness onset, 1188 had asymptomatic infection, and 503 reported symptoms lasting ≥4 weeks but had recovered at the time of the survey and were not queried about which symptom(s) persisted at least 4 weeks.
Figure 1.
Flowchart of study population. Abbreviations: COVID-19, coronavirus disease 2019; HCW, healthcare worker; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SARS-CoV-2–infected participants and nonparticipants were comparable with regard to sex (79.0% vs 77.6% of women), age distribution (48.4% vs 49.4% aged <40 years), and disease severity (1.2% vs 0.9% hospitalized and 0.4% vs 0.4% admitted to intensive care), according to data available in the centralized provincial database (data not shown).
The PCC4w analysis included 6061 HCWs with COVID-19, of whom 118 (2%) had been hospitalized and 5943 (98%) were nonhospitalized cases. The PCC12w analysis included 1783 HCWs (37 [2%] hospitalized and 1746 [98%] nonhospitalized).
For controls, 20 770 were called and 11 498 were reached. Among them, 1243 (11%) did not meet inclusion criteria, 2527 (22%) refused to participate, and 3338 (29%) did not complete the survey, resulting in 4390 controls for the analysis (Figure 1).
Compared to all SARS-CoV-2–infected HCWs reported provincially, case-participants were similar for sex (78% vs 79% women), age distribution (49% vs 48% aged <40 years), and percentage hospitalized (0.9% vs 1.2%).
Both hospitalized and nonhospitalized COVID-19 cases completed the questionnaire on average 10 weeks (median, 9 weeks [range, 5–35 weeks]) after illness onset. Compared to SARS-CoV-2–infected participants, controls were younger and more likely to be female, White, and working in occupations without direct patient care (Table 1).
Table 1.
Characteristics of Participants and Comparison Groups, Quebec Healthcare Workers
| Type of Participants | |||
|---|---|---|---|
| Characteristics | Symptomatic Hospitalized COVID-19 HCWs | Symptomatic Nonhospitalized COVID-19 HCWs | Non–COVID-19 HCWs (Controls) |
| Total No. | 118 | 5934 | 4390 |
| Weeks from illness onset or testing to study participation, mean ± SD | 10.3 ± 4.5 | 10.2 ± 4.3 | 9.9 ± 3.5 |
| Age, y, mean ± SD | 46.7 ± 11.9 | 40.0 ± 12.1 | 39.0 ± 10.4 |
| 18–29 | 12 (10.2) | 1410 (23.7) | 807 (18.4) |
| 30–39 | 21 (17.8) | 1547 (26.0) | 1750 (39.9) |
| 40–49 | 30 (25.4) | 1550 (26.1) | 1045 (23.8) |
| 50–59 | 39 (33.1) | 1102 (18.5) | 608 (13.9) |
| 60–80 | 16 (13.6) | 334 (5.6) | 180 (4.1) |
| Sex, female | 83 (70.3) | 4704 (79.2) | 3819 (87.0) |
| Race/ethnicity | |||
| White | 81 (68.6) | 4713 (79.3) | 3995 (91.0) |
| Black | 8 (6.8) | 482 (8.1) | 103 (2.4) |
| Hispanic | 10 (8.5) | 163 (2.7) | 68 (1.6) |
| Arab | 8 (6.8) | 176 (3.0) | 78 (1.8) |
| Asian | 2 (1.7) | 141 (2.4) | 56 (1.3) |
| Other/no response | 9 (7.6) | 268 (4.5) | 90 (2.1) |
| Occupation | |||
| Physician | 2 (1.7) | 241 (4.1) | 221 (5.0) |
| Nurse | 20 (17.0) | 1118 (18.8) | 900 (20.5) |
| Nurse assistant | 15 (12.7) | 469 (7.9) | 222 (5.1) |
| Patient healthcare assistant | 40 (33.9) | 1526 (25.7) | 523 (11.9) |
| Housekeeping | 5 (4.2) | 197 (3.3) | 40 (0.9) |
| Administration/manager | 13 (11.0) | 591 (9.9) | 618 (14.1) |
| Psychosocial worker | 5 (4.2) | 197 (3.3) | 397 (9.0) |
| Other | 18 (15.3) | 1585 (26.7) | 1469 (33.5) |
| Vaccination status | |||
| Unvaccinated | 114 (96.6) | 5484 (92.3) | 2980 (67.9) |
| 1 dose 0–13 d before illness onset | 2 (1.7) | 230 (3.9) | 280 (6.4) |
| 1 dose ≥14 d before illness onset | 2 (1.7) | 216 (3.6) | 926 (21.1) |
| 2 doses | 0 (0.0) | 13 (0.2) | 204 (4.7) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: COVID-19, coronavirus disease 2019; HCW, healthcare worker; SD, standard deviation.
Prevalence and Symptoms of PCC
Overall, 46.2% (95% CI, 45.0%–47.5%) of the nonhospitalized COVID-19 cases reported symptoms persisting ≥4 weeks (17.4% with at least 1 severe symptom), and 39.9% (95% CI, 38.3%–41.5%) had symptoms persisting ≥12 weeks. Among hospitalized COVID-19 cases, 76.3% (95% CI, 67.1%–82.5%) reported symptoms persisting ≥4 weeks (39.8% with ≥1 severe symptom) and 67.6% (95% CI, 57.7%–79.1%) had symptoms persisting ≥12 weeks (35.1% with ≥1 severe symptom) (Supplementary Table 1).
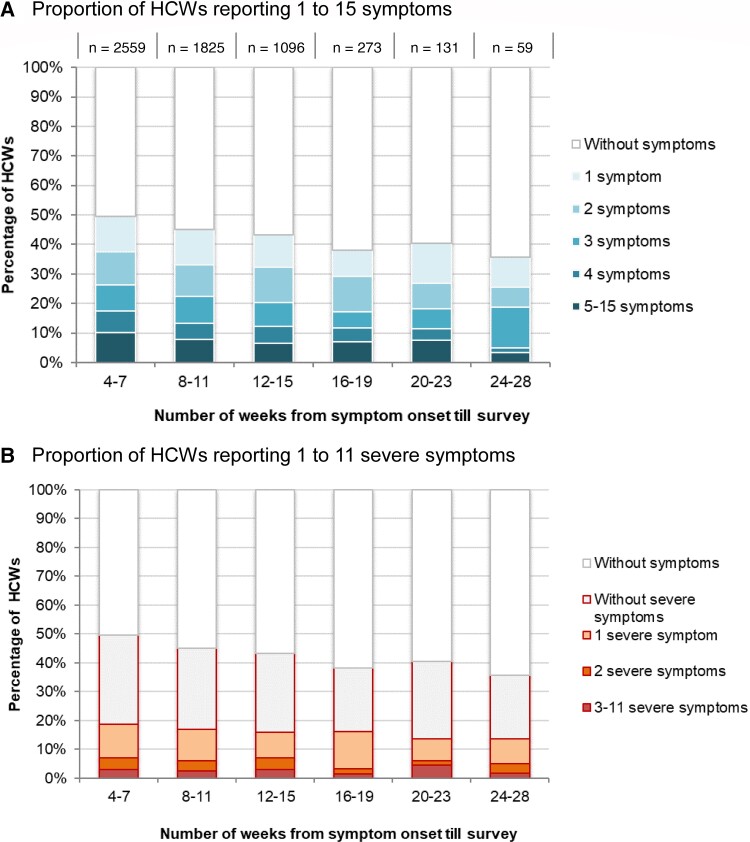
Persistent post–COVID-19 symptom prevalence among nonhospitalized cases decreased from 49.6% for HCWs reporting symptoms at 4–7 weeks post–COVID-19 onset to 36.6% at 24–27 weeks (trend test: P < .001). The proportion with ≥1 severe symptom (18.7% to 13.6%) showed little decline with time from illness onset (trend tests: P = .93) (Figure 2).
Figure 2.
Distribution of the number of symptoms (A) and number of severe symptoms (B) reported by nonhospitalized healthcare workers, by time from coronavirus disease 2019 illness onset to survey. Abbreviation: HCW, healthcare worker.
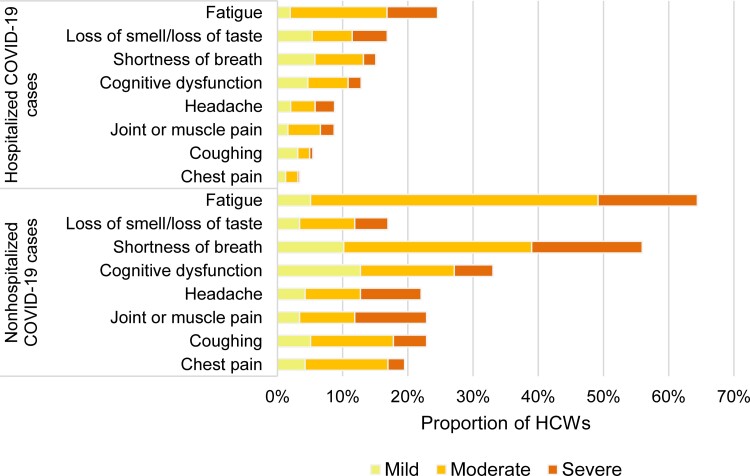
Among nonhospitalized and hospitalized cases, the most frequent symptoms lasting ≥4 weeks were fatigue (30% vs 64%), loss of smell or taste (20% vs 17%), shortness of breath (20% vs 56%), cognitive dysfunction (15% vs 33%), headache (13% vs 23%), and joint and muscular pain (10% vs 22%) (Figure 3). Symptoms more often rated as severe were loss of smell/taste, fatigue, and headache among nonhospitalized, and shortness of breath, headache, and joint and muscular pain among hospitalized cases (Figure 3). Among cases with persistent post–COVID-19 fatigue, 99% also reported pre–COVID-19 fatigue; the level of fatigue, however, was rated as mild by 84% pre–COVID-19 and moderate (63%) or severe (28%) post–COVID-19. The overall prevalence of these symptoms slowly decreased over time, but the prevalence of severe symptoms barely changed (Figure 3 and Supplementary Table 2).
Figure 3.
Prevalence and severity of the main symptoms still present ≥4 weeks after illness onset reported by nonhospitalized (A) and hospitalized (B) healthcare workers with coronavirus disease 2019. Cognitive dysfunction defined as self-reporting often or very often presenting difficulty to concentrate or maintain attention, difficulty to organize oneself, forgetting things, or losing necessary items among those who did not present with these dysfunctions before being infected with severe acute respiratory syndrome coronavirus 2. Abbreviations: COVID-19, coronavirus disease 2019; HCW, healthcare worker.
The correlation between symptoms was generally greater for symptoms of the same system (Supplementary Table 3). Loss of smell/taste was the only symptom negatively or not correlated with any other symptom.
Self-Reported Cognitive Dysfunctions and Psychological Distress
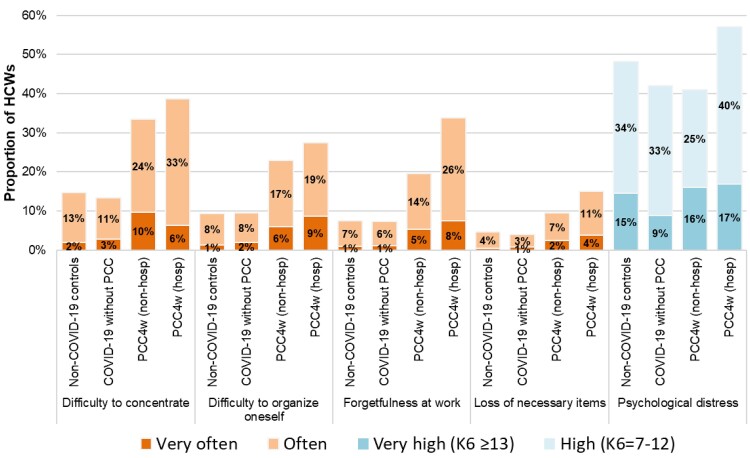
The prevalence of self-reported difficulty concentrating, difficulty organizing oneself, forgetfulness, and loss of necessary items often or very often was 33%, 23%, 20%, and 10%, respectively, among nonhospitalized PCC4w cases, compared to 39%, 28%, 34% and 15% among hospitalized PCC4w cases (Figure 4). These impairments were no less frequent when considering the 12-week definition.
Figure 4.
Prevalence of self-reported cognitive dysfunctions and psychological distress among non–coronavirus disease 2019 (COVID-19) controls, COVID-19 cases without post-COVID conditions (PCC), and nonhospitalized and hospitalized cases with PCC, among Quebec healthcare workers. Abbreviations: COVID-19, coronavirus disease 2019; HCW, healthcare worker; hosp, hospitalized; K6, Kessler Psychological Distress Scale; non-hosp, nonhospitalized; PCC, post-COVID conditions; PCC4w, ≥4-week post-COVID conditions; PCC12w, ≥12-week post-COVID conditions.
All 4 aspects of cognitive dysfunction were at least twice as frequent in PCC4w cases as in non–COVID-19 controls or in COVID-19 cases without physical persistent symptoms (Figure 4). In contrast, psychological distress was similarly reported as high or very high by PCC4w cases than by cases without PCC or by non–COVID-19 controls.
In multivariable models all aspects of cognitive dysfunction were associated with PCC4w, psychological distress, and fatigue (Table 2). They steadily increased with the level of psychological distress and the severity of fatigue (Supplementary Table 4 and Supplementary Figure 1). Difficulty concentrating, difficulty organizing oneself, forgetfulness, and losing necessary items were 2–3 times more common in PCC4w cases compared to non–COVID-19 controls; and 4–5 times more prevalent in individuals with very high psychological distress compared to those without distress. The 4 aspects of cognitive dysfunction were 3–4 times more frequent in those with severe fatigue compared to those without fatigue (Table 2).
Table 2.
Prevalence of Cognitive Dysfunctions by Clinical Status (Cases with ≥4-Week Post-COVID Conditions and Non-Coronavirus Disease 2019 Controls), Psychological Distress Level, Fatigue Level, and Demographic Characteristics and Adjusted Prevalence Ratios Among Quebec Healthcare Workers (N = 6962)
| Cognitive Dysfunction | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difficulty Concentratinga |
Difficulty Organizing Oneselfa |
Forgetfulnessa | Loss of Necessary Itemsa | |||||||||
| Evaluated Expositions | Prevalence, % | aPRb | (95% CI) | Prevalence, % | aPRb | (95% CI) | Prevalence, % | aPRb | (95% CI) | Prevalence, % | aPRb | (95% CI) |
| PCC | ||||||||||||
| Non–COVID-19 controls | 14.6 | ref | … | 9.3 | ref | … | 7.6 | ref | … | 4.7 | ref | … |
| ≥4-week PCC cases | 33.4 | 2.6 | (2.4–2.8) | 23.1 | 2.8 | (2.5–3.1) | 20.0 | 3.0 | (2.6–3.4) | 9.8 | 2.2 | (1.9–2.7) |
| Psychological distress | ||||||||||||
| No (K6 <7) | 8.1 | ref | … | 4.7 | ref | … | 4.1 | ref | … | 2.3 | ref | … |
| High (K6 = 7–12) | 24.1 | 2.4 | (2.1–2.8) | 15.9 | 2.7 | (2.3–3.3) | 13.7 | 2.7 | (2.2–3.4) | 7.3 | 2.4 | (1.8–3.1) |
| Very high (K6 ≥13) | 53.8 | 4.6 | (4.0–5.3) | 38.1 | 5.6 | (4.6–6.7) | 31.5 | 5.2 | (4.2–6.4) | 17.2 | 4.3 | (3.2–5.8) |
| Fatigue | ||||||||||||
| No or mild | 10.0 | ref | … | 6.7 | ref | … | 5.7 | ref | … | 2.6 | ref | … |
| Moderate | 21.1 | 1.8 | (1.6–2.1) | 13.2 | 1.7 | (1.4–2.0) | 10.8 | 1.7 | (1.4–2.0) | 6.0 | 2.0 | (1.5–2.7) |
| Severe | 47.0 | 2.8 | (2.4–3.2) | 33.8 | 2.9 | (2.5–3.5) | 29.9 | 3.1 | (2.6–3.8) | 16.7 | 3.8 | (2.8–5.1) |
| Sex | ||||||||||||
| Male | 15.8 | ref | … | 11.3 | ref | … | 9.7 | ref | … | 4.0 | ref | … |
| Female | 22.6 | 1.2 | (1.1–1.4) | 14.9 | 1.2 | (1.0–1.4) | 12.6 | 1.2 | (1.0–1.4) | 7.0 | 1.5 | (1.1–2.0) |
| Age, y | ||||||||||||
| 18–29 | 22.9 | ref | … | 15.1 | ref | … | 11.4 | ref | … | 8.0 | ref | … |
| 30–39 | 19.5 | 1.0 | (.9–1.1) | 13.5 | 1.1 | (.9–1.3) | 11.7 | 1.3 | (1.1–1.5) | 6.2 | 0.9 | (.7–1.2) |
| 40–49 | 24.6 | 1.1 | (1.0–1.3) | 15.7 | 1.1 | (.9–1.3) | 13.7 | 1.2 | (1.0–1.5) | 6.5 | 0.8 | (.7–1.1) |
| 50–59 | 22.2 | 1.0 | (.9–1.2) | 15.1 | 1.1 | (.9–1.3) | 12.9 | 1.2 | (1.0–1.5) | 6.6 | 0.9 | (.7–1.2) |
| 60–80 | 12.8 | 0.8 | (.4–1.0) | 7.7 | 0.7 | (.5–1.0) | 6.3 | 0.8 | (.5–1.1) | 4.2 | 0.7 | (.4–1.3) |
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence interval; COVID-19, coronavirus disease 2019; K6, Kessler Psychological Distress Scale; PCC, post-COVID condition; ref, reference category.
For each manifestation, “yes” corresponds to a reported current frequency of often or very often.
Robust Poisson regression models adjusted for sex, age (5 categories: 18–29, 30–39, 40–49, 50–59, ≥60 years), race/ethnicity (6 categories: White, Black, Hispanic, Arab, Asian, other), occupation (8 categories: physician, nurse, nurse assistant, patient healthcare assistant, housekeeping, administration/management, psychosocial worker, other), fatigue (3 categories: none or mild, moderate, severe), and psychological distress (3 categories: no, <7; high, 7–12; very high, ≥13 in the Kessler scale).
Risk Factors for PCC
PCC prevalence was higher in hospitalized compared to nonhospitalized acute COVID-19 cases, in those aged ≥40 years, and in females (Table 3, Supplementary Table 5, and Supplementary Figure 2). Only 4% of cases had been vaccinated with at least 1 dose ≥14 days before illness onset, and their risk compared to unvaccinated cases was lower for PCC4w (PR, 0.8 [95% CI, .7–.9]), but not PCC12w (PR, 0.9 [95% CI, .7–1.1]), although sample size was limited (Supplementary Table 5).
Table 3.
Risk Factors for Post-COVID Conditions Among Coronavirus Disease 2019 Healthcare Workers in Quebec, According to Varying Case Definitions (Multivariable Log-Binomial Regression Model)
| PCC Definition | ||||||
|---|---|---|---|---|---|---|
| ≥4-Week PCC (Any Symptom) |
≥4-Week PCC-S (≥1 Severe Symptom) |
≥12-Week PCC (Any Symptom) |
||||
| (n = 6061) | (n = 6061) | (n = 1783) | ||||
| Risk Factor | PR | (95% CI) | PR(n = 6061) | (95% CI) | PR | (95% CI) |
| Model 1: sociodemographic variables | ||||||
| Age, y (ref = 18–29 y) | ||||||
| 30–39 | 1.2 | (1.1–1.3) | 1.1 | (.9–1.3) | 1.2 | (1.0–1.5) |
| 40–49 | 1.4 | (1.3–1.5) | 1.4 | (1.2–1.7) | 1.5 | (1.3–1.8) |
| 50–59 | 1.4 | (1.2–1.5) | 1.3 | (1.1–1.5) | 1.3 | (1.0–1.5) |
| 60–80 | 1.3 | (1.1–1.5) | 1.1 | (.8–1.4) | 1.2 | (.9–1.6) |
| Sex (ref = male) | ||||||
| Female | 1.2 | (1.1–1.3) | 1.5 | (1.3–1.7) | 1.1 | (1.0–1.3) |
| Race/ethnicity (ref = White) | ||||||
| Black | 0.7 | (.6–.8) | 0.7 | (.5–.9) | 0.6 | (.5–.8) |
| Hispanic | 0.9 | (.7–1.0) | 1.0 | (.8–1.4) | 0.8 | (.6–1.2) |
| Arab | 0.7 | (.6–.9) | 0.8 | (.5–1.1) | 0.8 | (.6–1.1) |
| Asian | 0.7 | (.6–.9) | 0.7 | (.4–1.0) | 0.9 | (.6–1.4) |
| Other/no response | 1.0 | (.9–1.1) | 1.1 | (.9–1.4) | 1.0 | (.8–1.3) |
| Occupation (ref = admin) | ||||||
| Physician | 0.7 | (.6–.9) | 0.6 | (.4–.9) | 0.8 | (.5–1.1) |
| Nurse | 1.0 | (.9–1.1) | 1.1 | (.9–1.3) | 0.9 | (.7–1.1) |
| Nurse assistant | 1.1 | (1.0–1.2) | 1.4 | (1.1–1.7) | 1.0 | (.8–1.3) |
| Patient healthcare assistant | 1.0 | (.9–1.1) | 1.2 | (1.0–1.5) | 0.9 | (.8–1.1) |
| Housekeeping | 1.0 | (.8–1.2) | 1.1 | (.8–1.6) | 0.8 | (.5–1.2) |
| Psychosocial worker | 1.0 | (.8–1.2) | 0.8 | (.5–1.1) | 0.9 | (.7–1.2) |
| Other | 0.9 | (.8–1.0) | 0.8 | (.6–1.0) | 0.7 | (.6–.9) |
| Model 2: Vaccination adjusted for sociodemographic variables | ||||||
| Vaccination statusa (ref = unvaccinated) | ||||||
| 1 dose 0–13 d before illness onset | 0.9 | (.8–1.1) | 1.0 | (.7–1.3) | 0.9 | (.7–1.1) |
| ≥1 dose ≥14 d before illness onset | 0.8 | (.6–.9) | 0.8 | (.6–1.1) | 0.9 | (.7–1.1) |
| Model 3: Hospitalization adjusted for sociodemographic variables and vaccination status | ||||||
| Severity of acute disease (ref = nonhospitalized) | ||||||
| Hospitalized | 1.5 | (1.4–1.7) | 2.1 | (1.7–2.6) | 1.6 | (1.3–2.1) |
Abbreviations: admin, administration and management work; CI, confidence interval; PCC, post-COVID condition; PCC-S, post-COVID condition with at least 1 severe symptom; ref, reference; PR, prevalence ratio.
Vaccinated with 1 dose 0–13 days before illness onset or vaccinated with at least 1 dose ≥14 days before illness onset (13, 0, and 5 cases vaccinated with 2 doses for each PCC definition).
DISCUSSION
This study included >6000 hospitalized and nonhospitalized COVID-19 cases, representative of all laboratory-confirmed HCWs in Quebec, to estimate the prevalence of PCC using clinical information at the moment of the survey (completed 5–28 weeks after illness onset). COVID-19 symptoms persisting ≥4 weeks were reported by about three-quarters (76%) of hospitalized and nearly half (46%) of nonhospitalized COVID-19 cases with little decline at ≥12 weeks. Self-reported cognitive dysfunctions were highly prevalent and 2–3 times more frequent in PCC4w cases than controls, but were also strongly associated with psychological distress and fatigue.
Similar to our results, a meta-analysis of 63 studies estimated that 61% of laboratory-confirmed COVID-19 cases had symptoms lasting 4–12 weeks and 53% had symptoms lasting >12 weeks [3]. Most included studies were among hospitalized patients, had smaller sample size (between 58 and 1733 participants), and were at moderate or high risk of bias mainly due to participant selection and poor validity of outcome measures, according to the authors. Other studies among nonhospitalized patients have reported highly variable PCC prevalences, ranging from 13% to 44% for symptoms lasting ≥4 weeks [11–13], 5% to 35% for symptoms lasting ≥8 weeks [11, 13, 14], and 14% to 64% for symptoms lasting 5–8 months [15–21]. The estimation of PCC prevalence is limited by the difficulty in obtaining a representative sample of cases with participation and attrition being highly influenced by the persistence of symptoms [17, 22]. Our large cohort is likely representative of all HCWs with laboratory-confirmed COVID-19 in the province of Quebec, and self-selection based on symptom persistence may be limited because original recruitment was for a broader study of workplace exposure and prevention, nor PCC.
A staggering >100 physical, cognitive, and psychological symptoms have been reported in PCC cases [23]. Similar to our findings, several literature reviews found that fatigue, respiratory symptoms, and cognitive and mental health issues were most frequently reported [3, 22, 24–26]. Memory impairment, brain fog, poor attention, or difficulty thinking have been reported by other authors in 22%–88% of patients with lasting symptoms [5, 25, 27, 28], but these prevalences did not take into consideration the contribution of mood disorders or fatigue, highly associated with cognitive abnormalities and also features of PCC [6, 7]. Objectively measured cognitive deficits were reported among 46 patients 6 months after severe COVID-19, independent of mental health and fatigue [29]. We similarly observed that the prevalence of cognitive symptoms was 2–3 times higher among patients with PCCs compared to non–COVID-19 HCWs, whose baseline prevalence was as high as 14%.
While some studies found that COVID-19 patients frequently reported anxiety or depression [22, 24, 30], a systematic review including 33 studies found similar rates of anxiety or depression in the general population [31]. In our study, very high psychological distress, which is strongly associated with anxiety and depression [32, 33], was similarly common among PCC cases and controls and likely related to other work-related psychosocial risks and stressors afflicting HCWs during the pandemic [34]. The prognosis of these cognitive dysfunctions is unknown, but the lack of decline in prevalence over 4–28 weeks is worrisome. If persisting over the long term, this not infrequent sequela of COVID-19 could become both personally and professionally impactful on a significant scale among the highly infected population of HCWs. Our study also underscores the significant impact of fatigue and psychological distress during the pandemic on HCW cognitive function independent of PCC status, which also has important implications for quality healthcare delivery.
As reported elsewhere, our hospitalized cases had a higher prevalence and severity of PCC than ambulatory cases [14, 16, 22], with female sex and increasing age also associated with higher PCC risk [11, 14, 17, 18, 27, 35–37]. We could not properly assess the impact of vaccination, as few participants had been vaccinated before their COVID-19 illness.
Our study has several limitations. Using CDC and WHO case definitions for PCC based on 4-week or 12-week duration may have overestimated the prevalence of clinically significant PCC. Clinical significance may be improved by requiring longer duration but remains suboptimal in allowing any symptom of any intensity. Temporal patterns were identified using a single time survey completed at different intervals since illness onset and querying only about persisting ongoing symptoms and calculating later time from illness onset to survey. This likely limited recall bias and the selective attrition of less symptomatic patients in follow-up studies [3, 17], but may have underestimated the prevalence of symptoms for shorter intervals. Symptom description was based on subjective reporting. This allowed us to capture a large spectrum of the severity of the conditions, including less severe manifestations for which a medical consultation is not sought. A reporting bias is, however, possible considering that most symptoms are not specific and may be due to other conditions, as illustrated by the reported prevalence of fatigue before SARS-CoV-2 infection [38]. We did not collect information on some potentially relevant risk factors, such as comorbidity or initial COVID-19 symptoms and their severity [16, 17, 27, 35, 37]. Our questionnaire, designed in the fall of 2020, did not collect data on insomnia or postexertional malaise later, also considered to be PCC-qualifying symptoms [23]. Cognitive functions were not objectively assessed with validated tools, and self-reporting might have overestimated their prevalence. Physical symptoms among non–COVID-19 controls were not collected. Comparing the prevalence but also the severity and persistence of symptoms among cases and controls would have allowed better characterization of PCC and PCC-associated cognitive and psychological symptoms. It should be considered in future research. We cannot rule out the possibility of selection bias as half of reached HCWs refused to participate (20%) or did not complete the survey (30%). This may overestimate the PCC prevalence if HCWs with persisting symptoms had participated more. However, self-selection bias may be small as participants were invited to participate to a larger study on workplace prevention practices and not on PCCs. Our participants were mostly unvaccinated working adults infected by the original SARS-CoV-2 virus: findings may not be generalizable to children, elderly adults, vaccinated individuals, or those infected by subsequent variants of concern. Despite these limitations, this study shows the high burden of COVID-19 sequelae among nonhospitalized adults.
In conclusion, PCC may be a frequent sequela of ambulatory COVID-19 in working-age adults, with important effects on cognition. With so many HCWs infected since the beginning of the COVID-19 pandemic, the ongoing implications for quality healthcare delivery could be profound should cognitive dysfunction and other severe PCC symptoms persist in a professionally disabling way over the longer term.
Supplementary Material
Contributor Information
Sara Carazo, Centre Hospitalier Universitaire de Québec–Laval University Research Center, Quebec City, Quebec, Canada; Biological and Occupational Risks Unit, Institut national de santé publique du Québec, Quebec City, Quebec, Canada.
Danuta M Skowronski, Communicable Diseases and Immunization Services, BC Centre for Disease Control, Vancouver, British Columbia, Canada.
Robert Laforce, Jr, Interdisciplinary Memory Clinic, Department of Neurological Sciences, Centre Hospitalier Universitaire de Quebec, Quebec City, Quebec, Canada; Faculty of Medicine, Laval University, Quebec City, Quebec, Canada.
Denis Talbot, Centre Hospitalier Universitaire de Québec–Laval University Research Center, Quebec City, Quebec, Canada; Social and Preventive Medicine Department, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada.
Emilia L Falcone, Department of Medicine, Faculty of Medicine, University of Montreal, Montreal, Quebec, Canada; Center for Inflammation, Immunity and Infectious Diseases, Montreal Clinical Research Institute, Montreal, Quebec, Canada.
Denis Laliberté, Social and Preventive Medicine Department, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada; Centre Intégré Universitaire en Santé et Services Sociaux de la Capitale-Nationale, Quebec City, Quebec, Canada.
Geoffroy Denis, Centre intégré universitaire de santé et de services sociaux du Centre-Sud de l'Île de Montréal, Montreal, Quebec, Canada; Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Quebec, Canada.
Pierre Deshaies, Centre Intégré Universitaire en Santé et Services Sociaux de Chaudière-Appalaches, Lévis, Quebec, Canada.
Sandrine Hegg-Deloye, Centre Hospitalier Universitaire de Québec–Laval University Research Center, Quebec City, Quebec, Canada.
Gaston De Serres, Centre Hospitalier Universitaire de Québec–Laval University Research Center, Quebec City, Quebec, Canada; Biological and Occupational Risks Unit, Institut national de santé publique du Québec, Quebec City, Quebec, Canada; Social and Preventive Medicine Department, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Jasmin Villeneuve, Richard Martin (Institut national de santé du Québec), Armelle Lorcy (Centre Hospitalier Universitaire de Québec–Université Laval Research Center), Francine Ducharme (Université de Montréal), and Bianka Paquet-Bolduc (Institut Universitaire en cardiologie et pneumologie de Québec) for their contribution to the large study conducted to evaluate COVID-19 workplace exposure, infection risk, and prevention in healthcare workers in Quebec, and all the healthcare workers that participated to this study.
Financial support. This work was supported by the Ministère de la santé et des services sociaux du Québec.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Flong-term-effects.html. Accessed 10 January 2022.
- 2. World Health Organization . A clinical case definition of post COVID-19 condition by a Delphi consensus. 2021:20. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1. Accessed 10 January 2022.
- 3. Domingo FR, Waddell LA, Cheung AM, et al. . Prevalence of long-term effects in individuals diagnosed with COVID-19: an updated living systematic review. medRxiv [Preprint]. Posted online 6 June 2021. Accessed 10 January 2022.
- 4. Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open 2021; 4:e2111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis HE, Assaf GS, McCorkell L, et al. . Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Golan D, Doniger GM, Wissemann K, et al. . The impact of subjective cognitive fatigue and depression on cognitive function in patients with multiple sclerosis. Mult Scler J 2018; 24:196–204. [DOI] [PubMed] [Google Scholar]
- 7. Ortelli P, Ferrazzoli D, Sebastianelli L, et al. . Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: insights into a challenging symptom. J Neurol Sci 2021; 420:117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Serres G, Carazo S, Villeneuve J, et al. . Enquête épidémiologique sur les travailleurs de la santé atteints par la COVID-19: rapport d’étape pour la période du 12 juillet 2020 au 16 janvier 2021. Institut national de santé publique du Québec. 2021. https://www.inspq.qc.ca/sites/default/files/publications/3137-enquete-epidemiologique-travailleurs-sante-atteints-covid19.pdf. Accessed 5 September 2021.
- 9. Pratt LA, Dey AN, Cohen AJ. Characteristics of adults with serious psychological distress as measured by the K6 scale: United States, 2001–04. Adv Data 2007:382:1–18. [PubMed] [Google Scholar]
- 10. Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol 2013; 177:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sudre CH, Murray B, Varsavsky T, et al. . Attributes and predictors of long COVID. Nat Med 2021; 27:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blair PW, Brown DM, Jang M, et al. . The clinical course of COVID-19 in the outpatient setting: a prospective cohort study. Open Forum Infect Dis 2021; 8:ofab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Havervall S, Rosell A, Phillipson M, et al. . Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA 2021; 325:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darley DR, Dore GJ, Cysique L, et al. . Persistent symptoms up to four months after community and hospital-managed SARS-CoV-2 infection. Med J Aust 2021; 214:279–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein H, Asseo K, Karni N, et al. . Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clin Microbiol Infect 2021; 27:769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun 2021; 12:6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Augustin M, Schommers P, Stecher M, et al. . Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021; 6:100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 2021; 76:405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen MS, Kristiansen MF, Hanusson KD, et al. . Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis 2021; 73:e4058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacobson KB, Rao M, Bonilla H, et al. . Patients with uncomplicated coronavirus disease 2019 (COVID-19) have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis 2021; 73:e826–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Logue JK, Franko NM, McCulloch DJ, et al. . Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 2021; 4:e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iqbal A, Iqbal K, Arshad Ali S, et al. . The COVID-19 sequelae: a cross-sectional evaluation of post-recovery symptoms and the need for rehabilitation of COVID-19 survivors. Cureus 2021; 13:e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayes LD, Ingram J, Sculthorpe NF. More than 100 persistent symptoms of SARS-CoV-2 (long COVID): a scoping review. Front Med 2021; 8:750378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malik P, Patel K, Pinto C, et al. . Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—a systematic review and meta-analysis. J Med Virol 2022; 94:253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Michelen M, Manoharan L, Elkheir N, et al. . Characterising long COVID: a living systematic review. BMJ Glob Health 2021; 6:e005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. . More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021; 11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Desgranges F, Tadini E, Munting A, et al. . Post-COVID-19 syndrome in outpatients: a cohort study. J Gen Intern Med 2022; 37:1943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graham EL, Clark JR, Orban ZS, et al. . Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers.” Ann Clin Transl Neurol 2021; 8:1073–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hampshire A, Chatfield DA, MPhil AM, et al. . Multivariate profile and acute-phase correlates of cognitive deficits in a COVID-19 hospitalised cohort. EClinicalMedicine 2022; 47:101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lamontagne SJ, Winters MF, Pizzagalli DA, Olmstead MC. Post-acute sequelae of COVID-19: evidence of mood and cognitive impairment. Brain Behav Immun Health 2021; 17:100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bourmistrova NW, Solomon T, Braude P, Strawbridge R, Carter B. Long-term effects of COVID-19 on mental health: a systematic review. J Affect Disord 2022; 299:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kessler RC, Andrews G, Colpe LJ, et al. . Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 2002; 32:959–76. [DOI] [PubMed] [Google Scholar]
- 33. Furukawa TA, Kessler RC, Slade T, Andrews G. The performance of the K6 and K10 screening scales for psychological distress in the Australian National Survey of Mental Health and Well-Being. Psychol Med 2003; 33:357–62. [DOI] [PubMed] [Google Scholar]
- 34. Carazo S, Pelletier M, Talbot D, Jauvin N, De Serres G, Vézina M. Psychological distress of healthcare workers in Québec (Canada) during the second and the third pandemic waves. J Occup Environ Med 2022; 64:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tenforde MW, Kim SS, Lindsell CJ, et al. . Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. MMWR Morb Mortal Wkly Rep 2020; 69:993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-acute sequelae of COVID-19 (PASC) or long COVID: a meta-analysis and systematic review [manuscript published online ahead of print 16 April 2022]. J Infect Dis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Munblit D, Bobkova P, Spiridonova E, et al. . Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy 2021; 51:1107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lerner AM, Robinson DA, Yang L, et al. . Toward understanding COVID-19 recovery: National Institutes of Health workshop on postacute COVID-19. Ann Intern Med 2021; 174:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.