Figure 3.
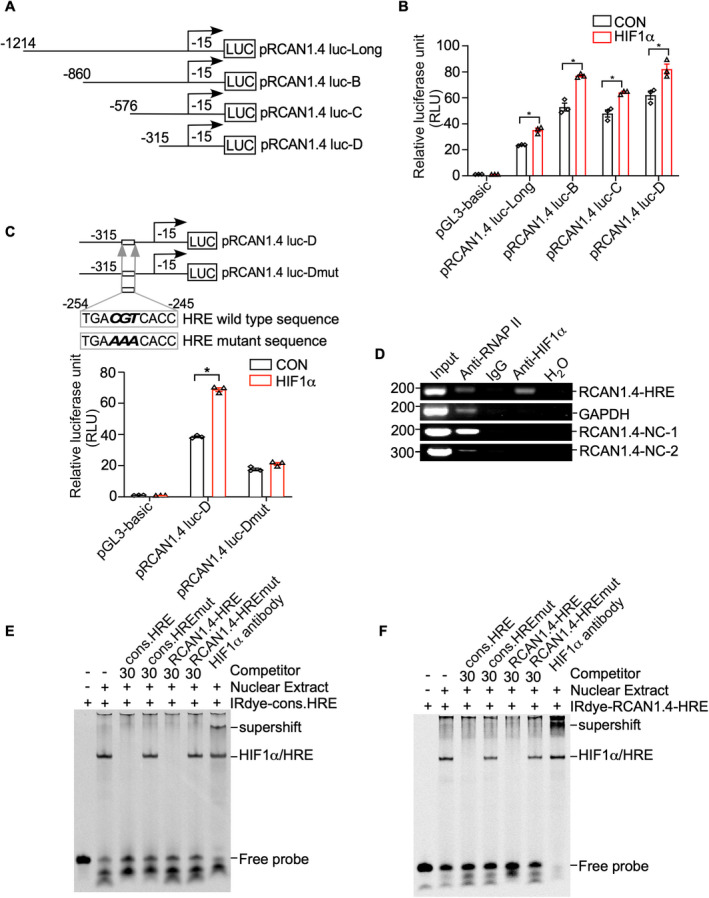
Identification of the HRE site in the RCAN1.4 promoter fragment. (A) Schematic diagrams of human RCAN1.4 promoter truncation constructs. Four different lengths of the 5′‐flanking region of the human RCAN1.4 promoter fragment were cloned into the pGL3‐basic plasmid upstream of the luciferase reporter gene (Luc). The arrow indicates the direction of transcription. The numbers represent the end points of each construct. (B) RCAN1.4 promoter truncation constructs were transfected into HEK293 cells with either the HIF1α expression plasmid (pHA‐HIF1α) or an empty vector. A dual luciferase assay was performed 48 h after transfection. pGL3‐basic was used as the negative control. (C) Comparison of the mutant and wild‐type sequences of the putative RCAN1.4‐HRE site in the RCAN1.4 promotor region. pRCAN1.4 luc‐D and pRCAN1.4 luc‐Dmut were transfected with the HIF1α expression vector or empty vector into HEK293 cells. pGL3‐basic was used as a negative control. A dual luciferase assay was performed 48 h after transfection. (D) DNA and protein from HEK293 cells were cross‐linked and immunoprecipitated with anti‐RNA polymerase II antibody (anti‐RNAPII), anti‐HIF1α antibody, and antimouse IgG, respectively. The nonimmunoprecipitated sample was used as an input. PCR was performed with the input, precipitated chromatin samples, and H2O to amplify a short DNA fragment spanning the putative RCAN1.4‐HRE site in the RCAN1.4 promoter region (panel 1), a short fragment in the GAPDH gene (panel 2), a nonspecific DNA fragment upstream RCAN1.4‐HRE (RCAN1.4‐NC‐1, panel 3) and a nonspecific DNA fragment downstream RCAN1.4‐HRE (RCAN1.4‐NC‐2, panel 4). Input and anti‐RNA polymerase II antibodies (anti‐RNAPII) were used as positive controls. IgG and H2O were used as negative controls. (E) EMSA was carried out with IRDye800‐labeled consensus HRE oligonucleotides (IRDye‐cons. HRE). The free probe is shown in lane 1. The addition of the nuclear extract shifted the probe band (lane 2). Competition assays were performed with a 30‐fold excess of unlabeled consensus HRE (cons. HRE, lane3), mutant consensus HRE (cons. HREmut, lane 4), putative RCAN1.4 promoter HRE (RCAN1.4‐HRE, lane 5), and mutant putative RCAN1.4 promoter HRE (RCAN1.4‐HREmut, lane 6). A supershift assay was carried out with an anti‐HIF1α antibody (lane 7). (F) IRDye800‐labeled HRE site in the human RCAN1.4 promoter (IRDye‐RCAN1.4‐HRE) was employed. The free probe is shown in lane 1. The addition of nuclear extract shifted the probe band (lane 2). Competition assays were carried out with a 30‐fold excess of unlabeled consensus HRE (cons. HRE, lane3), mutant consensus HRE (cons. HREmut, lane 4), and putative RCAN1.4 promoter HRE (RCAN1.4‐HRE, lane 5), and mutant putative RCAN1.4 promoter HRE (RCAN1.4‐HREmut, lane 6). A supershift assay was carried out with an anti‐HIF1α antibody (lane 7). Values represent means ± SEM (n = 3), *p < 0.05, as calculated by two‐way ANOVA with Bonferroni's multiple comparison post hoc test. HRE, hypoxia‐responsive element; RCAN1.4, the isoform 4 of regulator of calcineurin 1.
