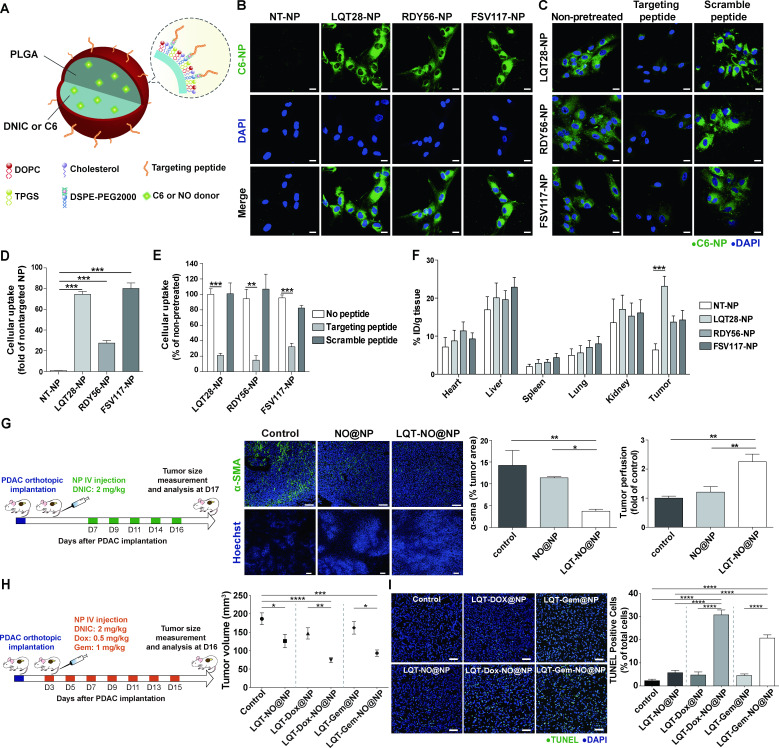
Figure 5.
Tumour stroma-targeted lipid-PLGA NPs exhibited enhanced PDAC tumour uptake and NO delivery capability to reprogramme the desmoplastic tumour stroma in PDAC. (A) Structural schematic of NPs modified with tumour stroma-targeted peptides. (B) Primary, culture-activated human PSCs were treated with coumarin 6 (C6)-loaded NPs (0.175 µg/mL) modified with the tumour stroma-targeted peptide LQT28, RDY56 or FSV117 for 1 hour. Scale bars, 20 µm. Green, coumarin 6-loaded NPs; blue, nuclei (DAPI). (C) The uptake of NPs modified with the tumour stroma-targeted peptide LQT28, RDY56 or FSV117 into human PSCs was competitively inhibited by the addition of free corresponding stroma-targeted peptide. Cells were treated with free peptides for 30 mins prior to treatment with NPs and analysed for fluorescence signals by confocal microscopy. Scale bars, 20 µm. Green, coumarin 6-loaded NPs; blue, nuclei (DAPI). (D) The cellular uptake of NPs was imaged and quantified using a Zeiss LSM 780 confocal microscope (n=4). (E) The cellular uptake of NPs with or without pretreatment with free peptides was imaged and quantified using a Zeiss LSM 780 confocal microscope (n=4). (F) The tissue distributions of C6 in different formulations (n=7–15). NPs modified with LQT28 peptides showed enhanced tissue uptake in tumours 4- hour after intravenous administration in the orthotopic AK4.4 PDAC model. (G) Seven days after the implantation of AK4.4 PDAC cells and mice were treated with LQT-NO@NPs or non-targeted NO@NPs (DNIC: 2 mg/kg) on days 7, 9, 11, 14 and 16; tumours were then analysed on day 17 by immunostaining. Representative immunofluorescence images and quantification of α-SMA-positive cells and Hoechst 33 342-positive cells in PDAC tumours after treatment with LQT-NO@NPs or non-targeted NO@NPs in a murine orthotopic (AK4.4) PDAC model. Green, α-SMA-positive myofibroblasts; blue, DAPI (top panel) or Hoechst 33 342 (bottom panel) (n=5 section images from three mice). Scale bars, 100 µm. Mice were injected intravenously with 500 µg of Hoechst 33342 on day 17, after which the tumours were harvested. (H) Three days after the implantation of AK4.4 PDAC cells and mice were treated with DNIC (2 mg/kg) and/or Dox (0.5 mg/kg) or Gem (1 mg/kg) loaded in lipid-PLGA NPs modified with LQT28 on days 3, 5, 7, 9, 11, 13 and 15; tumours were then analysed on day 16. Volumes of orthotopic PDAC tumours 16 days post implantation in treated and untreated (control) mice (n=7–8), DNIC: 2 mg/kg; Dox: 0.5 mg/kg; Gem: 1 mg/kg. (I) Representative immunofluorescence images and quantification of TUNEL staining after treatment with different formulations in a murine orthotopic (AK4.4) PDAC model, as described in (h) (n=8 section images from four mice). Scale bar, 50 µm. All data are shown as the mean±SEM. *P<0.05, **p<0.01, ***p<0.001. DNIC, dinitrosyl iron complexes; IV, intravenously; NO, nitric oxide; NP, nanoparticle; PDAC, pancreatic ductal adenocarcinoma; PLGA, poly(lactic-coglycolic) acid; PSC, pancreatic stellate cells; SMA, smooth muscle actin.