FIG 2.
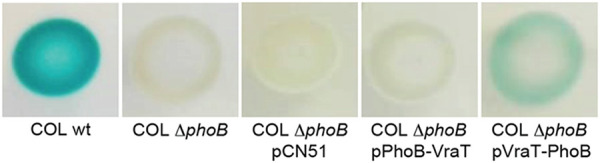
C-terminal domain of VraT is extracellular. VraT topology was assessed by fusing the phoB gene to vraT at either end. The phoB fusions to vraT were expressed from the pCN51 plasmid, under the control of a cadmium-inducible promoter, in the background of a phoB deletion mutant (COLΔphoB). An empty pCN51 plasmid was also introduced into COLΔphoB (COLΔphoB + pCN51) and used as a negative control. The wild-type strain COL was used as positive control. The strains were grown on plates containing cadmium chloride and chromogenic BCIP (5-bromo-4-chloro-3-indolyl phosphate), a substrate which, when processed by the alkaline phosphatase PhoB, an enzyme active only in the extracytoplasmic environment, gives a blue coloration to the colonies (41). Only COL and COLΔphoB carrying the vraT-phoB fusion (COLΔphoB + pVraT-PhoB) showed alkaline phosphatase activity, indicating that the C-terminal domain but not the N-terminal domain of VraT faces the extracytoplasmic environment.
