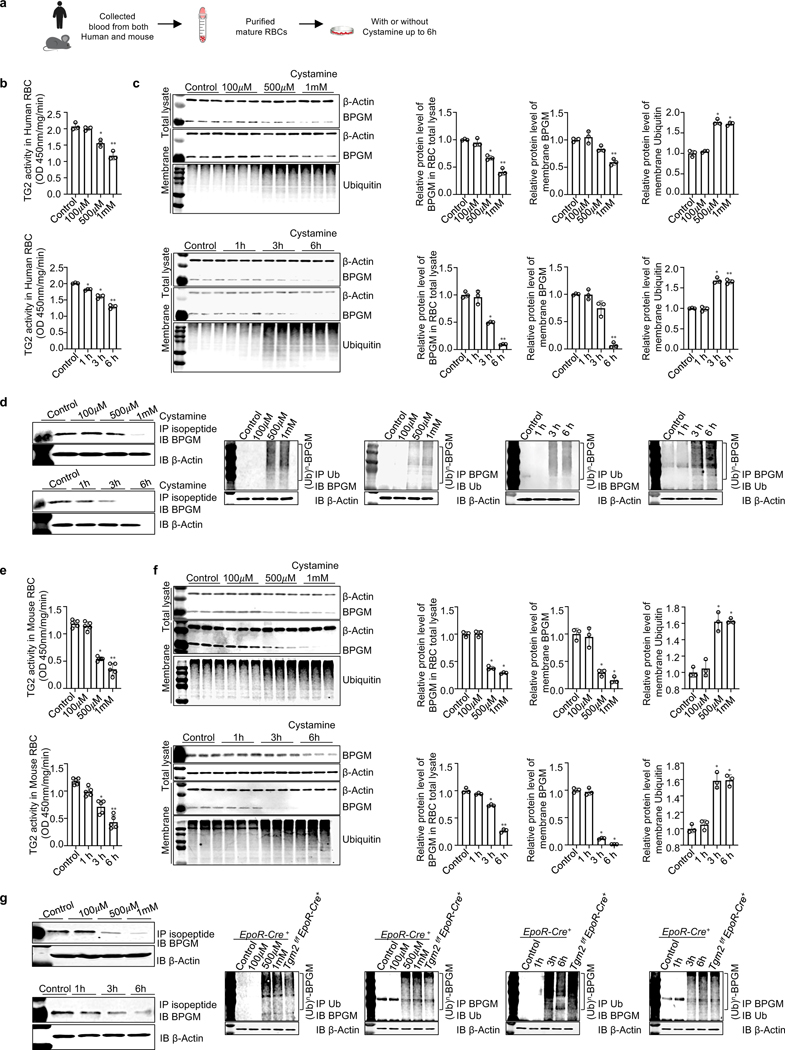
Figure 2. TG2-mediated PTM stabilizes erythrocyte BPGM and maintains protein homeostasis by competing against polyubiquitination and degradation.
a. Schematic representation of experiment design.
b. In human erythrocytes, TG2 activity was reduced by cystamine treatment in a dose (upper panel) and time (lower panel) dependent manner (n=3).
c. In vitro inhibitive effect of cystamine on TG2-mediated isopeptide modification on BPGM protein and proteins modified with ubiquitin in cultured human erythrocytes are dose-dependent (upper panel) and time-dependent (lower panel). Protein bands were normalized to untreated erythrocytes (n=3).
d. Isopeptide modification of BPGM mediated by TG2 was reduced by cystamine treatment in a dose and time dependent manner in cultured human erythrocytes (left panel). IP BPGM followed by western blot against ubiquitin and vice versa (right panels).
e. In mouse erythrocytes, TG2 activity was reduced by cystamine treatment in a dose (upper panel) and time (lower panel) dependent manner (n=5).
f. Effect of cystamine on BPGM protein and proteins modified with ubiquitin in mouse RBCs is in a dose (upper panel) and time (lower panel) dependent manner, followed with quantification of protein bands by densitometry (n=3).
g. IP isopeptide followed by western blot against BPGM; IP BPGM followed by western blot against ubiquitin and vice versa. Data are expressed as mean ± SD. For different dosage treatment, *P<0.05 versus control, **P<0.05 versus 500 μM. For different time points, *P<0.05 versus control, **P<0.05 versus 3h. Data were analyzed by one-way ANOVA followed with Sidak’s multiple comparisons test. See also Figure S2.