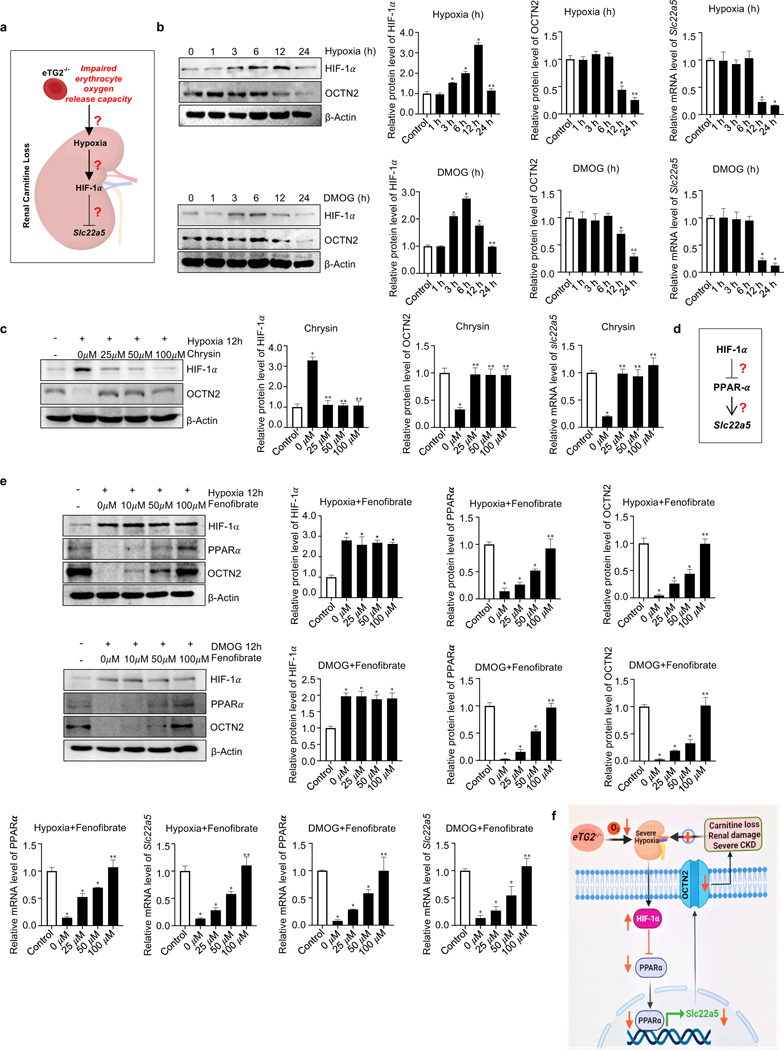
Figure 6. Hypoxia-mediated downregulation of OCTN2 via HIF-1α-PPARα axis is a key molecular mechanism linking eTG2 ablation to Ang II-induced carnitine loss, renal damage and severe CKD.
a. Schematic hypothesis linking renal hypoxia with HIF-1α induction and reduced Slc22a5 transcription.
b. Hypoxia (1% O2, upper panel) or DMOG (1mM, lower panel) treatment directly induced HIF1-α and reduced OCTN2 protein levels in a time-dependent manner in cultured murine kidney explants. Protein bands were normalized to control group. qRT-PCR analyses of Slc22a5 mRNA levels in kidney samples from the same treatment was followed with protein quantification. *P<0.05 versus Control, **P<0.05 versus 12h, (n=3).
c. Induction of HIF-1α and reduction of OCTN2 protein levels and Slc22a5 mRNA levels were attenuated by Chrysin treatment in cultured murine renal explants. *P<0.05 versus Control, **P<0.05 versus 0 μM Chrysin, (n=3).
d. Hypothesis that PPARα functions downstream of HIF-1α underlying hypoxia-mediated reduction in Slc22a5 transcription.
e. Fenofibrate, a PPARα agonist, restored hypoxia (upper panel) or DMOG-mediated (lower panel) reduction of OCTN2 and PPARα proteins and their mRNA levels in a dosage-dependent manner in cultured mouse kidney explants. *P<0.05 versus Control, **P<0.05 versus 50 μM Fenofibrate, (n=3).
f. Signaling pathways linking eTG2 deficiency to renal damage and CKD: Decreased erythrocyte O2 offload in eTG2−/− mice leads to renal hypoxia and reduction of renal Slc22a5 transcription and decreased OCTN2 via HIF1α-PPARα axis. Without intervention, renal hypoxia, reduced OCTN2 levels and carnitine loss function as a malicious positive feed forward loop to promote severe hypoxia, excess carnitine losses, renal damage and severe CKD progression. All data are expressed as mean ± SD and were analyzed by two-way ANOVA followed with Tukey’s multiple comparisons test.