Abstract
Oxidized lipids derived from omega-6 (n-6) and omega-3 (n-3) polyunsaturated fatty acids, collectively known as oxylipins, are bioactive signaling molecules that play diverse roles in human health and disease. Supplementation with n-3 docosahexaenoic acid (DHA) during pregnancy has been reported to decrease the risk of preterm birth in singleton pregnancies, which may be due to effects of DHA supplementation on oxylipins or their precursor n-6 and n-3 fatty acids. There is only limited understanding of the levels and trajectory of changes in plasma oxylipins during pregnancy, effects of DHA supplementation on oxylipins and unesterified fatty acids, and whether and how oxylipins and their unesterified precursor fatty acids influence preterm birth. In the present study we used liquid chromatography-tandem mass spectrometry to profile oxylipins and their precursor fatty acids in the unesterified pool using plasma samples collected from a subset of pregnant Australian women who participated in the ORIP (Omega-3 fats to Reduce the Incidence of Prematurity) study. ORIP is a large randomized controlled trial testing whether daily supplementation with n-3 DHA can reduce the incidence of early preterm birth compared to control. Plasma was collected at study entry (≈pregnancy week 14) and again at ≈week 24, in a subgroup of 48 ORIP participants—12 cases with spontaneous preterm (<37 weeks) birth and 36 matched controls with spontaneous term (≥40 weeks) birth. In the combined preterm and term pregnancies, we observed that in the control group without DHA supplementation unesterified AA and AA-derived oxylipins 12-HETE, 15-HETE and TXB2 declined between weeks 14–24 of pregnancy. Compared to control, DHA supplementation increased unesterified DHA, EPA, and AA, DHA-derived 4-HDHA, 10-HDHA and 19,20-EpDPA, and AA-derived 12-HETE at 24 weeks. In exploratory analysis independent of DHA supplementation, participants with concentrations above the median for 5-lipoxygenase derivatives of AA (5-HETE, Odds Ratio (OR) 8.2; p = 0.014) or DHA (4-HDHA, OR 8.0; p = 0.015) at 14 weeks, or unesterified AA (OR 5.1; p = 0.038) at 24 weeks had higher risk of spontaneous preterm birth. The hypothesis that 5-lipoxygenase-derived oxylipins and unesterified AA could serve as mechanism-based biomarkers predicting spontaneous preterm birth should be evaluated in larger, adequately powered studies.
Keywords: Oxylipins, Plasma, Docosahexaenoic, Arachidonic, Linoleic, Preterm, Development
1. Introduction
Oxidized lipids derived from polyunsaturated fatty acids (PUFAs), collectively known as oxylipins, comprise a large, heterogeneous family of labile, bioactive signaling molecules that play diverse roles in human health and disease [1]. Oxylipins derived from arachidonic acid (AA), docosahexaenoic acid (DHA) and linoleic acid (LA) are reported to play pivotal regulatory roles in physiological processes that are relevant for pregnancy—including immune activation [2,3], resolution of inflammation [4,5], endothelial cell activation [6], and coagulation [7]—and have been proposed to play a role in gestational age at birth [8]. Most notably, 2-series prostaglandins (e.g. PGE2 and PGF2α) are well-known to play pivotal roles in initiation and progression of labor by promoting cervical maturation and myometrial contraction [9,10].
The placenta in preterm birth is characterized by an inflammatory phenotype [11], including alterations in prostanoid synthesis and catabolism [12], and women who delivered preterm had higher AA in maternal red blood cells and trophoblast tissue than women who delivered at term [13]. Human blood, placenta and fetal tissues are enriched in n-3 and n-6 fatty acids, and selected oxylipins derived from AA, DHA, and LA have been measured in cord blood [8,14]. Moreover, gene variants in fatty acid desaturase (FADS) enzymes involved in AA and DHA synthesis are associated with gestation duration among women with spontaneous labor [15].
Thus, it is possible that in addition to prostanoids, other bioactive oxylipins derived from AA (e.g. leukotrienes) [3,16,17], n-3 fatty acids, and/or LA could impact parturition; however, this line of inquiry has not been fully investigated. DHA is well-known to be concentrated in fetal tissues and the developing brain [18] and DHA supplementation in pregnancy has been investigated as a strategy for enhancing neurodevelopment [19,20] and decreasing the risk of preterm birth [21,22]. Several, but not all, studies reported that DHA supplementation decreased the risk of preterm birth and/or increased the risk of post-term births requiring obstetric intervention (induction or caesarean section) [19,21,23]. Such a shift in the curve of gestation length may be due to partial replacement of AA by DHA in placental oxylipin precursor pools [24–26], with subsequent reduction in PGE2 and uterine contractility. However, the effect of DHA supplementation on unesterified AA or PGE2 has not yet been demonstrated in pregnant women. DHA supplementation may also decrease inflammatory processes implicated in preterm birth by increasing the production of DHA-derived oxylipins with anti-inflammatory and pro-resolving properties in placenta or blood [27].
Most studies have focused on the role of AA, DHA and their metabolites during pregnancy. Little is known about LA or its metabolites (oxidized linoleic acid metabolites; OXLAMs), which have been mechanistically linked to several pathological conditions (reviewed in [28]). The combination of relatively high concentrations of OXLAMs in lipoproteins and strong expression of scavenger receptors in human placenta [29–31], provides a mechanism for delivery of OXLAMs to placenta [31,32], particularly during periods of inflammation and/or oxidative stress (reviewed in [33]) Feeding heated safflower oil (source of OXLAMs) to rats in early pregnancy had teratogenic effects [34]. However, the effects of OXLAM exposures on pregnancy are understudied in mammals [35].
Together, these observations indicate that exposures to oxylipins derived from DHA, AA, and LA in pregnancy could potentially play an important role in pregnancy. However, there is currently only limited understanding of whether and how oxylipins influence biochemical and clinical endpoints in pregnancy. Here we take an initial step toward filling these gaps by profiling oxylipins and their precursor fatty acids using plasma samples collected from a subset of pregnant Australian women enrolled in the ORIP (Omega-3 fats to Reduce the Incidence of Prematurity) study, a large randomized controlled trial testing whether daily supplementation with n-3 DHA can reduce the incidence of early preterm birth in Australian women. The ORIP team collected plasma from a subgroup of participants at study entry (<20 weeks of gestation, median 14 weeks) and again at pregnancy week 24, providing a unique opportunity to: (1) describe the levels and trajectory of changes in plasma unesterified oxylipins and precursor fatty acids in early pregnancy and near the threshold for viability of pregnancy; (2) determine the impact of DHA supplementation on oxylipins and precursor fatty acids; and (3) explore whether differences in oxylipins and precursor fatty acids at pregnancy weeks 14 and 24 hold promise as biomarkers for predicting risk of spontaneous preterm birth. This analysis is restricted to spontaneous births because obstetrical interventions (i.e. induced births, Caesarean sections) may occur for multiple reasons, some of which may be unrelated to endogenous biochemical mediators. Given the small size of the ORIP plasma subgroup, the latter exploration is anticipated to inform sample size calculations for larger, more adequately powered studies testing associations between these variables and spontaneous preterm birth.
2. Materials and methods
2.1. ORIP study design
A protocol paper [22] and primary outcome [23] detailing the main ORIP study have been previously published. Briefly, ORIP is a large randomized controlled trial testing whether daily supplementation with long-chain omega-3 (n-3) fatty acids (docosahexaenoic acid (DHA) 800mg plus eicosapentaenoic acid (EPA) 100mg) provided from trial entry (<20 weeks of gestation) until 34 weeks or delivery (whichever occurred first), can reduce the incidence of early preterm (<34 weeks) and preterm (<37 weeks) birth and other secondary maternal and fetal endpoints, compared to control. The baseline and demographic characteristics of the plasma subgroup (n = 48) analyzed here and the full ORIP cohort of randomized participants (n = 5,544) are shown in Table 1. Compared to the full ORIP cohort, the plasma subgroup tended to be slightly older, less racially diverse, more likely to drink alcohol, less likely to be primiparous, and more likely to have a previous preterm delivery. The ORIP study [23] showed that DHA supplementation did not decrease risk of early preterm birth (primary endpoint) or preterm birth (key secondary endpoint) in the total population. However, there was a significant reduction in the risk of preterm birth in singleton pregnancies (a prespecified subgroup analysis).
Table 1.
Baseline characteristics of pregnancies in the plasma subgroup and the full ORIP trial.
| Characteristic | Plasma subgroup Case (N = 12) | Control (N = 36) | n-3 Group (N = 27) | Control Group (N = 21) | Total (N = 48) | ORIP trial n-3 Group (N = 2770) | Control Group (N = 2774) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age (yr): Median (IQR) | 33.0 (31.0–34.2) | 33.0 (30.0–33.2) | 33.0 (30.0–34.0) | 33.0 (31.0–34.0) | 33.0 (30.8–34.0) | 30.0 (26.0–34.0) | 30.0 (27.0–33.0) |
| Gestation (wk): Median (IQR) | 13.4 (12.4–15.1) | 13.4 (12.7–14.6) | 13.4 (12.6–15.1) | 13.1 (12.4–14.6) | 13.4 (12.5–14.8) | 14.1 (12.7–16.4) | 14.1 (12.7–16.6) |
| Primiparous: n/N (%) | 3/12 (25.0) | 9/36 (25.0) | 6/27 (22.2) | 6/21 (28.6) | 12/48 (25.0) | 1223/2754 (44.4) | 1209/2765 (43.7) |
| Pregnancy with multiple fetuses: n/N (%) | 1/12 (8.3) | 0/36 (0.0) | 0/27 (0.0) | 1/21 (4.8) | 1/48 (2.1) | 52/2698 (1.9) | 48/2721 (1.8) |
| Weight (kg): Median (IQR) | 67.2 (63.6–71.6) | 67.5 (57.8–73.8) | 70.6 (57.6–73.5) | 66.9 (60.1–73.0) | 67.2 (58.0–73.4) | 69.7 (60.9–81.5) | 69.0 (60.9–81.0) |
| Caucasian race: n/N (%) | 10/12 (83.3) | 32/36 (88.9) | 24/27 (88.9) | 18/21 (85.7) | 42/48 (87.5) | 2057/2764 (74.4) | 2041/2767 (73.8) |
| Completed a high-school education: n/N (%) | 11/12 (91.7) | 31/36 (86.1) | 23/27 (85.2) | 19/21 (90.5) | 42/48 (87.5) | 2246/2756 (81.5) | 2194/2764 (79.4) |
| Smoked cigarettes at trial entry or leading up to pregnancy: n/N (%) | 0/12 (0.0) | 7/36 (19.4) | 4/27 (14.8) | 3/21 (14.3) | 7/48 (14.6) | 435/2755 (15.8) | 430/2766 (15.5) |
| Drank alcohol at trial entry or leading up to pregnancy: n/N (%) | 10/12 (83.3) | 26/36 (72.2) | 20/27 (74.1) | 16/21 (76.2) | 36/48 (75.0) | 1513/2755 (54.9) | 1562/2764 (56.5) |
| Previous preterm birth <37 weeks of gestation: n/N (%) | 3/12 (25.0) | 2/36 (5.6) | 4/27 (14.8) | 1/21 (4.8) | 5/48 (10.4) | 185/2754 (6.7) | 184/2766 (6.7) |
| Consumed dietary supplements containing n-3 long-chain polyunsaturated fatty acid in the previous 3 months: n/N (%) | 4/12 (33.3) | 3/36 (8.3) | 2/27 (7.4) | 5/21 (23.8) | 7/48 (14.6) | 374/2770 (13.5) | 368/2774 (13.3) |
| DHA level (% of total fatty acids in capillary whole blood): Median (IQR) | 2.8 (2.1–3.2) | 2.7 (2.5–3.0) | 2.7 (2.5–3.0) | 2.7 (2.4–3.1) | 2.7 (2.4–3.1) | 2.7 (2.3–3.1) | 2.7 (2.3–3.1) |
| Male fetus: n/N(%)* | 9/13 (69.2) | 19/36 (52.8) | 15/27 (55.6) | 13/22 (59.1) | 28/49 (57.1) | 1395/2758 (50.6) | 1409/2770 (50.9) |
DHA denotes docosahexaenoic acid, IQR denotes interquartile range. N is the total number of pregnancies unless otherwise indicated.
N is the total number of fetuses.
2.2. Sample collection, processing and selection
The protocol was approved by the Institutional Review Board of the Women’s and Children’s Health Network and written informed consent was obtained for all blood collection procedures. Following an overnight fast, venipuncture of the median cubital vein in the antecubital fossa was performed with an 18-gauge butterfly needle, with venous whole blood collected into 5 mL ‘purple top’ potassium-EDTA tubes. Whole blood was stored for up to 120 min at room temperature, followed by centrifugation. Plasma was carefully pipetted into each aliquot with care taken not to disturb the buffy coat. Samples were stored at −80 °C in Adelaide and shipped with ample dry ice and tracking information for analysis in the National Institutes of Health in Bethesda, Maryland, USA. Samples were analyzed in a de-identified, blinded manner, without knowledge of treatment group or case versus control status. Since each of these participants provided two samples (≈14 and 24 weeks), a total of 96 samples were analyzed.
2.3. Quantitation of oxylipins and precursor fatty acids in plasma
For liquid chromatography tandem mass spectrometry oxylipin assays, we applied the same methods and criteria as published in our recent detailed methods manuscript [1], adapted to target oxylipins that may be linked to pregnancy and parturition and with addition of unesterified fatty acids. In this method, human plasma samples were deproteinized with ice-cold methanol and extracted with solid-phase extraction. The separation was performed on a reverse phase ZorBAX RRHD Eclipse Plus C18 column using gradient programs consisting of 0.02% acetic acid and ammonium acetate buffer in water and acetonitrile mobile phases. Detection was achieved using the Qtrap 5500 system in electrospray negative ion mode with scheduled multiple reaction monitoring (sMRM).
2.4. Experimental design and matching strategy
To explore the hypothesis that an imbalance between unesterified LA, AA, and DHA and their respective oxylipin derivatives could create a biochemical predisposition to preterm delivery we matched the 12 participants who provided plasma at both of these time points and went on to have spontaneous preterm (<37 weeks) birth with 36 controls who went on to have spontaneous term (40+0–40+6 weeks) birth. The two groups were matched on maternal age and parity. Treatment group was not considered in the matching process, as the trial was still blinded at the time of selection for the plasma subgroup. Exact matching was performed first on parity and then age was matched using the nearest neighbor approach with Mahalanobis distance allowing for 1.5 SD of distance [36], resulting in a matched age range within 3 years.
2.5. Data analysis
Compounds were only reported if values were above the limit of quantitation (LOQ) in at least half of the samples with LOQ defined as a signal-to-noise ratio of >5. All profiled analytes and LOQs are provided in Table S1. In the main analysis, we use only the oxylipin samples that are not below LOQ. In the sensitivity analyses reported in the supplementary appendix (Tables S2–S5), we impute the values below the LOQ by substituting one half of the LOQ value for each respective plasma oxylipin. For the precursor unesterified fatty acids, all values were above the LOQ.
The aim of the first analysis was to determine if there was a significant change in the plasma fatty acid concentrations between weeks 14 and 24 separately in the control group and DHA group through a Wilcoxon matched-pairs signed-ranks test. The aim of the second analysis was to determine the difference in plasma fatty acid concentrations between the DHA and control groups at week 24 through a nonparametric local-linear regression controlling for the plasma fatty acid concentration at week 14. Each regression had 200 bootstrap replications. The aim of the third analysis was to explore if the plasma unesterified fatty acid or oxylipin concentrations could be biomarkers for spontaneous preterm birth. To account for non-linear associations, this analysis was performed conservatively by analyzing the sample according to groups of below and above median concentrations at each time point. Odds ratios of the above median group versus the below median group were calculated for each plasma oxylipin or fatty acid at each time point using logistic regressions adjusted for age and parity. Analyses were performed using Stata 15. Statistical significance was assessed at the p < 0.05 level with no adjustment for multiple comparisons due to the exploratory nature of the study.
3. Results
We investigated the levels and trajectory of changes of plasma oxylipins and precursor (unesterified) fatty acids during pregnancy, by measuring concentrations of these variables at 14 weeks and 24 weeks (shortly before the gestational limit for viable pregnancy) in the participants (n = 21) from the control group without DHA supplementation. Within-group changes in precursor fatty acids and oxylipin concentrations in the control group are shown in Table 2. Without DHA supplementation, we observed that unesterified concentrations of three n-6 fatty acids (AA, docosatetraenoic acid, and gamma-linolenic acid) and one n-9 fatty acid (mead acid) significantly declined during pregnancy. However, there was no evidence of any changes in n-3 fatty acids. Concentrations of three AA-derived oxylipins—12-HETE, 15-HETE and TXB2—also significantly declined between weeks 14–24 of pregnancy, however no changes were observed for DHA-derivatives or other oxylipins.
Table 2.
Changes in unesterified precursor fatty acids and oxylipins between weeks 14 and 24 in pregnancy without DHA supplementation (n = 21)
| Week 14 | Week 24 | p-value* | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
|
| |||
| Omega-6 fatty acids and oxylipins | |||
| LA** | 7.6 (4.8, 11) | 7.3 (4.1, 9.9) | .24 |
| 9-HODE | 4.2 (1.4, 11) | 3.4 (1.3, 7.9) | .42 |
| 13-HODE | 11 (3.4, 23) | 7.6 (5, 21) | .54 |
| 9,10-DiHOME | 2.4 (1.1, 5.1) | 1.6 (1.1, 4.3) | .56 |
| 13H-9,10E-LA | .25 (.16, .48) | .22 (.16, .36) | .73 |
| 9H-12,13E-LA | .16 (.067, .38) | .16 (.12, .24) | .93 |
| 9,12,13-TriHOME | .12 (.088, .19) | .14 (.098, .17) | .95 |
| GLA | .086 (.044, .13) | .049 (.036, .074) | .030 |
| DGLA | .12 (.086, .15) | .094 (.077, .11) | .059 |
| AA | .18 (.14, .25) | .14 (.12, .17) | .011 |
| 5-HETE | .17 (.14, .23) | .16 (.12, .17) | .08 |
| 12-HETE | .13 (.1, .24) | .088 (.08, .12) | .039 |
| 15-HETE | .29 (.26, .5) | .21 (.17, .22) | .001 |
| TxB2 | .11 (.066, .22) | .053 (.039, .11) | .048 |
| DTA | .045 (.034, .067) | .035 (.028, .048) | .033 |
| DPA n-6 | .042 (.033, .067) | .044 (.036, .05) | .45 |
| Omega-3 fatty acids and oxylipins | |||
| ALA | .44 (.23, .79) | .26 (.18, .51) | .08 |
| SDA (18:4 n-3) | .012 (.0061, .016) | .0058 (.0045, .0093) | .11 |
| ETA (20:4 n-3) | .014 (.011, .018) | .012 (.0089, .014) | .15 |
| EPA | .093 (.086, .1) | .083 (.056, .099) | .12 |
| DPA n-3 | .024 (.015, .038) | .023 (.016, .032) | .91 |
| DHA | .65 (.43, .75) | .54 (.4, .78) | .97 |
| 4-HDHA | .066 (.042, .09) | .05 (.03, .076) | .57 |
| 10-HDHA | .026 (.021, .035) | .019 (.014, .038) | .11 |
| 14-HDHA | .12 (.085, .22) | .078 (.059, .12) | .06 |
| 17-HDHA | .17 (.13, .23) | .12 (.1, .19) | .55 |
| 19,20-EpDPA | .032 (.018, .047) | .034 (.019, .063) | .31 |
| Other | |||
| Mead | .016 (.011, .02) | .011 (.0073, .013) | .003 |
Based on the Wilcoxon matched-pairs signed-ranks test.
9,10-EpOME & 9,10,13-TriHOME had >50% values below LOQ. Concentrations: fatty acids (ug/mL); oxylipins (ng/mL).
Concentrations of unesterified fatty acids and oxylipins in the DHA supplementation group in pregnancy weeks 14 and 24 are shown in Table 3. With DHA supplementation, within-group analysis suggested that unesterified concentrations of three n-3 fatty acids (EPA, DPAn-3, and DHA) and one n-6 fatty acid (DPAn-6) increased, while LA (18:2n-6), GLA (18:3n-6) and SDA (18:4n-3) decreased. Unesterified AA did not change. Two DHA-derived oxylipins (4-HDHA and 19,20-EpDPA) increased and one AA-derivative (15-HETE) decreased. Importantly, these within-group changes in this DHA cohort reflect a combination of effects of DHA supplementation and normal trajectory of change, and as such should be interpreted cautiously.
Table 3.
Changes in unesterified precursor fatty acids and oxylipins between weeks 14 and 24 in pregnancy with DHA supplementation (n=27).
| Week 14 | Week 24 | p-value* | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
|
| |||
| Omega-6 fatty acids and oxylipins | |||
| LA** | 8.5 (6.4, 13) | 7.6 (4.8, 11) | .038 |
| 9-HODE | 4.4 (2.7, 6.8) | 2.6 (1.1, 6.2) | .26 |
| 13-HODE | 8.4 (5.6, 15) | 5.6 (2.6, 13) | .15 |
| 9,10-DiHOME | 2 (1.1, 2.6) | 1.3 (.64, 4.1) | .61 |
| 13H-9,10E-LA | .25 (.21, .37) | .19 (.12, .31) | .21 |
| 9H-12,13E-LA | .19 (.13, .24) | .1 (.075, .28) | .85 |
| 9,12,13-TriHOME | .13 (.09, .22) | .11 (.086, .17) | .26 |
| GLA | .083 (.05, .13) | .05 (.028, .075) | <0.001 |
| DGLA | .096 (.081, .13) | .098 (.082, .12) | .71 |
| AA | .15 (.12, .18) | .18 (.13, .21) | .20 |
| 5-HETE | .19 (.14, .28) | .14 (.12, .19) | .012 |
| 12-HETE | .21 (.1, .28) | .17 (.1, .23) | .36 |
| 15-HETE | .33 (.25, .6) | .19 (.12, .32) | <0.001 |
| TxB2 | .098 (.06, .22) | .057 (.036, .14) | .16 |
| DTA | .04 (.03, .055) | .035 (.028, .048) | .54 |
| DPA n-6 | .04 (.03, .055) | .054 (.042, .085) | <0.001 |
| Omega-3 fatty acids and oxylipins | |||
| ALA | .59 (.3, .82) | .29 (.18, .51) | <0.001 |
| SDA (18:4 n-3) | .011 (.007, .02) | .008 (.004, .014) | .008 |
| ETA (20:4 n-3) | .012 (.009, .02) | .014 (.011, .019) | .28 |
| EPA | .075 (.049, .1) | .14 (.11, .19) | <0.001 |
| DPA n-3 | .02 (.014, .03) | .031 (.022, .04) | .023 |
| DHA | .62 (.47, .8) | 1.1 (.84, 1.6) | <0.001 |
| 4-HDHA | .07 (.04, .12) | .12 (.069, .17) | .004 |
| 10-HDHA | .03 (.024, .041) | .034 (.021, .072) | .058 |
| 14-HDHA | .19 (.15, .31) | .2 (.12, .31) | .71 |
| 17-HDHA | .19 (.12, .3) | .21 (.15, .28) | .60 |
| 19,20-EpDPA | .03 (.02, .05) | .077 (.057, .11) | <0.001 |
| Other | |||
| Mead | .01 (.008, .018) | .009 (.006, .016) | .041 |
Based on the Wilcoxon matched-pairs signed-ranks test.
9,10-EpOME & 9,10,13-TriHOME had >50% values below LOQ.
Concentrations: fatty acids (ug/mL); oxylipins (ng/mL).
In order to determine the effects of DHA supplementation on plasma oxylipins and precursor fatty acids, we compared levels of each variable at week 24 in the DHA group versus the control group in the combined term and preterm pregnancies, with each variable adjusted for baseline values. We observed that DHA supplementation significantly increased concentrations of unesterified n-3 DHA, DPA(n-3), EPA, as well as n-6 AA and DPAn-6, but did not alter linoleic acid, alpha-linolenic acid, or other fatty acids (Table 4). DHA supplementation also significantly increased three DHA-derived oxylipins (4-HDHA, 10-HDHA and 19,20-EpDPA), and one AA-derived oxylipin (12-HETE).
Table 4.
DHA supplementation vs Control at week 24.
| Control (n=21) | DHA (n=27) | Nonparametric ANCOVA* | ||
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Difference (95% CI) | p-value | |
|
| ||||
| Omega-6 fatty acids and oxylipins | ||||
| LA** | 7.3 (4.1, 9.9) | 7.6 (4.8, 11) | .64 (−1.1, 2.6) | .50 |
| 9-HODE | 3.4 (1.3, 7.9) | 2.6 (1.1, 6.2) | −.58 (−4.7, 4.4) | .78 |
| 13-HODE | 7.6 (5, 21) | 5.6 (2.6, 13) | −1.3 (−11, 8.3) | .77 |
| 9,10-DiHOME | 1.6 (1.1, 4.3) | 1.3 (.64, 4.1) | .39 (−2, 2.9) | .74 |
| 13H-9,10E-LA | .22 (.16, .36) | .19 (.12, .31) | −.029 (−.22, .18) | .77 |
| 9H-12,13E-LA | .16 (.12, .24) | .1 (.075, .28) | .028 (−.19, .26) | .80 |
| 9,12,13-TriHOME | .14 (.098, .17) | .11 (.086, .17) | −.021 (−.089, .041) | .53 |
| GLA | .049 (.036, .074) | .05 (.028, .075) | −.000018 (−.018, .016) | .99 |
| DGLA | .094 (.077, .11) | .098 (.082, .12) | .016 (−.00089, .031) | .052 |
| AA | .14 (.12, .17) | .18 (.13, .21) | .037 (.015, .062) | .002 |
| 5-HETE | .16 (.12, .17) | .14 (.12, .19) | .02 (−.052, .098) | .58 |
| 12-HETE | .088 (.08, .12) | .17 (.1, .23) | .071 (.0082, .13) | .036 |
| 15-HETE | .21 (.17, .22) | .19 (.12, .32) | .023 (−.055, .1) | .55 |
| TxB2 | .053 (.039, .11) | .057 (.036, .14) | .011 (−.051, .069) | .73 |
| DTA | .035 (.028, .048) | .035 (.028, .048) | .0051 (−.0038, .013) | .25 |
| DPA n-6 | .044 (.036, .05) | .054 (.042, .085) | .024 (.013, .037) | <0.001 |
| Omega-3 fatty acids and oxylipins | ||||
| ALA | .26 (.18, .51) | .29 (.18, .51) | .021 (−.1, .13) | .71 |
| SDA (18:4 n-3) | .0058 (.0045, .0093) | .0079 (.004, .014) | .0013 (−.0019, .004) | .40 |
| ETA (20:4 n-3) | .012 (.0089, .014) | .014 (.011, .019) | .0025 (−.0013, .0055) | .16 |
| EPA | .083 (.056, .099) | .14 (.11, .19) | .072 (.049, .099) | <0.001 |
| DPA n-3 | .023 (.016, .032) | .031 (.022, .04) | .008 (−.00029, .015) | .035 |
| DHA | .54 (.4, .78) | 1.1 (.84, 1.6) | .56 (.36, .78) | <0.001 |
| 4-HDHA | .05 (.03, .076) | .12 (.069, .17) | .099 (.011, .21) | .048 |
| 10-HDHA | .019 (.014, .038) | .034 (.021, .072) | .019 (.0037, .036) | .010 |
| 14-HDHA | .078 (.059, .12) | .2 (.12, .31) | .12 (−.0085, .25) | .06 |
| 17-HDHA | .12 (.1, .19) | .21 (.15, .28) | .019 (−.15, .16) | .81 |
| 19,20-EpDPA | .034 (.019, .063) | .077 (.057, .11) | .033 (.01, .055) | .002 |
| Other | ||||
| Mead | .011 (.007, .013) | .009 (.006, .016) | .0028 (−.0005, .006) | .09 |
Difference is estimated using a nonparametric ANCOVA controlling for the mediator at Week 14. A negative difference means the DHA group had a lower concentration than the control group.
9,10-EpOME & 9,10,13-TriHOME had >50% values below LOQ. Concentrations: fatty acids (ug/mL); oxylipins (ng/mL).
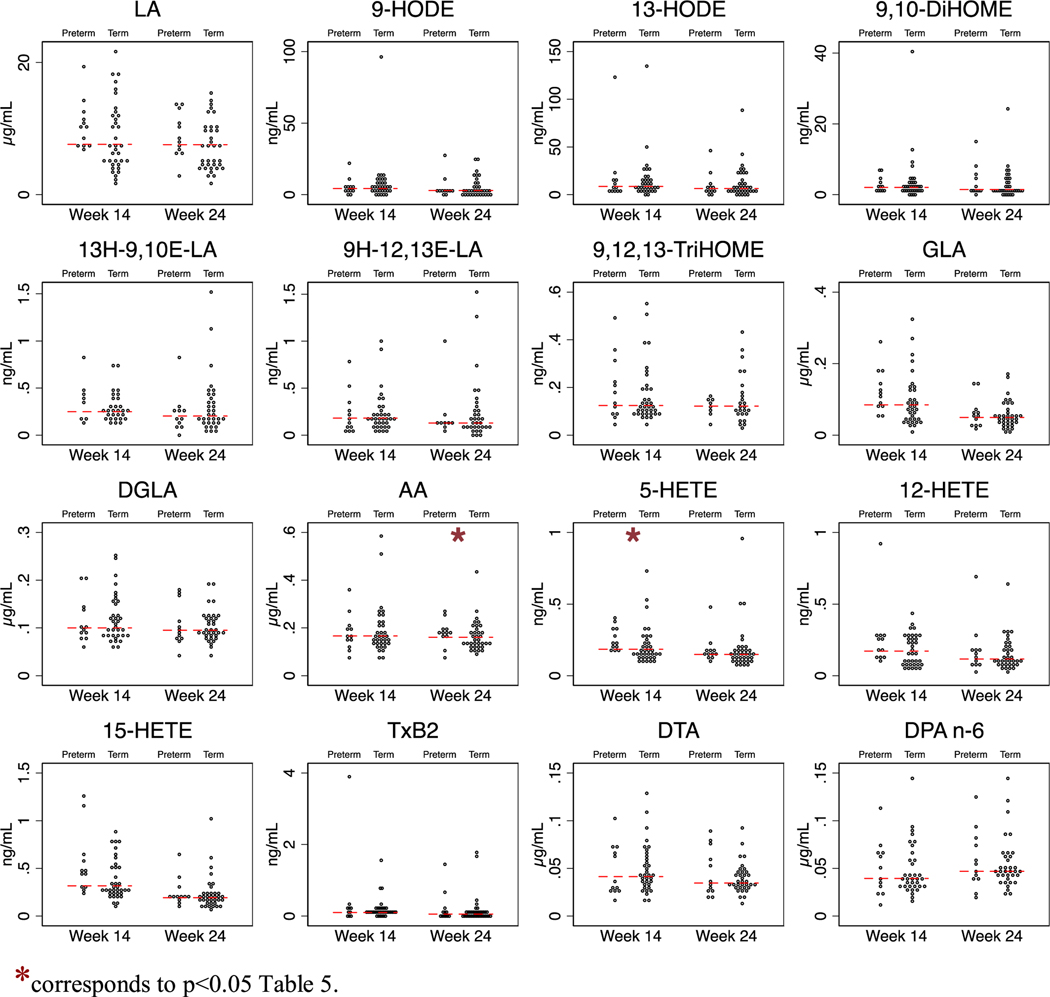
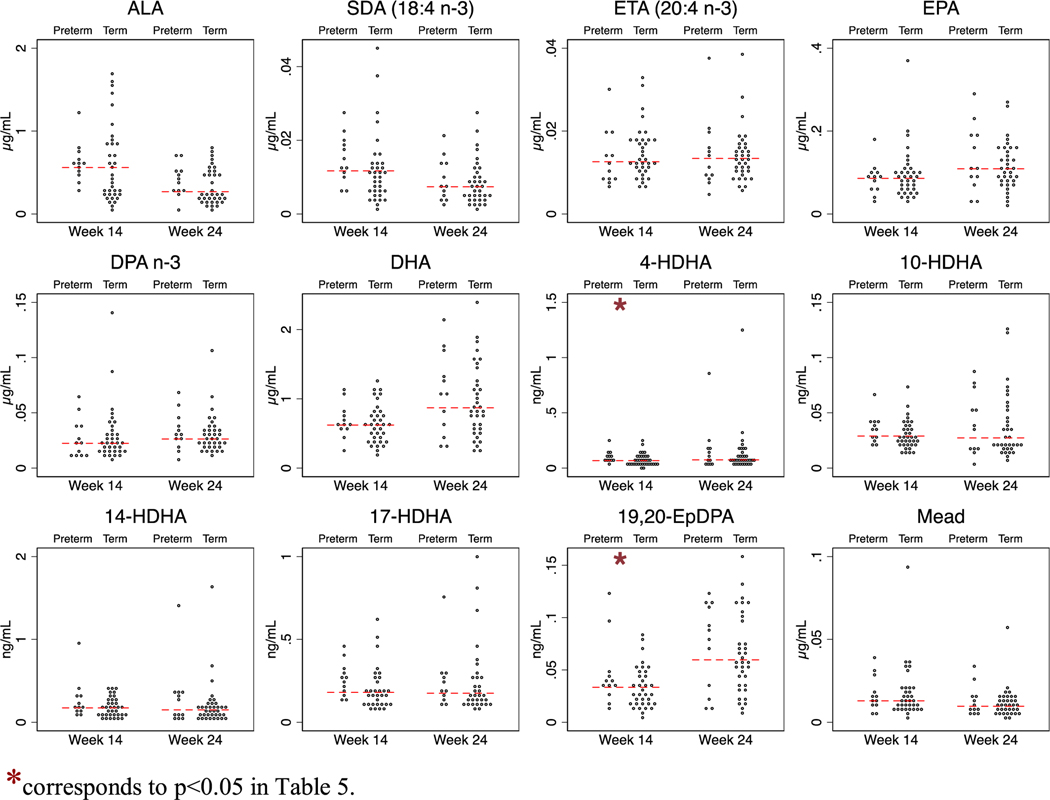
To explore whether differences in precursor fatty acids at week 14 (initial visit) or 24 (shortly before the gestational limit for viable pregnancy) hold promise as biomarkers for predicting risk of preterm birth, we compared the proportion of cases (spontaneous preterm births, n = 12) vs controls (spontaneous matched term births, n = 36), independent of DHA supplementation, that were above the median level at 14 and 24 weeks using logistic regression adjusting for age and parity. This analysis showed that participants with concentrations of unesterified AA above the median at 24 weeks had higher risk of spontaneous preterm birth (Odds ratio (OR) 5.1; p = 0.038) (Table 5 and Figure 1 and 2). None of the other fatty acids were predictive of spontaneous preterm birth at week 14 or 24.
Table 5.
Risk of spontaneous preterm birth according to concentrations of oxylipins & precursor fatty acids (n =48 including 12 cases of spontaneous preterm birth (25 %)).
| Week 14 (n = 48) | Week 24 (n = 48) | |||||||
|---|---|---|---|---|---|---|---|---|
| Below | Above | Odds | p-value | Below | Above | Odds | p-value | |
| Median | Median | Ratio | Median | Median | Ratio | |||
|
| ||||||||
| Omega-6 fatty acids and oxylipins | ||||||||
| LA** | 17% | 35% | 2.8 | .14 | 21% | 30% | 1.7 | .43 |
| 9-HODE | 39% | 14% | 0.2 | .05 | 22% | 26% | 1.2 | .75 |
| 13-HODE | 26% | 26% | 1.0 | .98 | 29% | 21% | 0.6 | .49 |
| 9,10-DiHOME | 25% | 25% | 1.0 | .99 | 24% | 26% | 1.1 | .87 |
| 13H-9,10E-LA | 16% | 28% | 2.0 | .41 | 27% | 23% | 0.8 | .70 |
| 9H-12,13E-LA | 25% | 26% | 1.1 | .92 | 24% | 19% | 0.7 | .62 |
| 9,12,13-TriHOME | 17% | 33% | 2.5 | .18 | 24% | 25% | 1.1 | .93 |
| GLA | 17% | 35% | 2.8 | .14 | 21% | 30% | 1.7 | .44 |
| DGLA | 33% | 17% | 0.4 | .20 | 29% | 22% | 0.7 | .64 |
| AA | 24% | 27% | 1.2 | .79 | 12% | 39% | 5.1 | .038 |
| 5-HETE | 8% | 42% | 8.2 | .014 | 21% | 29% | 1.6 | .49 |
| 12-HETE | 21% | 29% | 1.6 | .50 | 21% | 29% | 1.6 | .50 |
| 15-HETE | 12% | 38% | 4.4 | .050 | 17% | 33% | 2.7 | .16 |
| TxB2 | 13% | 32% | 3.7 | .10 | 25% | 25% | 1.0 | .97 |
| DTA | 29% | 22% | 0.7 | .53 | 29% | 22% | 0.7 | .58 |
| DPA n-6 | 25% | 26% | 1.1 | .94 | 25% | 26% | 1.1 | .91 |
| Omega-3 fatty acids and oxylipins | ||||||||
| ALA | 17% | 35% | 2.9 | .13 | 17% | 35% | 2.8 | .15 |
| SDA (18:4 n-3) | 15% | 38% | 3.6 | .07 | 25% | 26% | 1.1 | .93 |
| ETA (20:4 n-3) | 29% | 22% | 0.6 | .51 | 25% | 26% | 1.0 | .94 |
| EPA | 21% | 30% | 1.7 | .46 | 25% | 26% | 1.1 | .93 |
| DPA n-3 | 25% | 26% | 1.0 | .99 | 21% | 30% | 1.7 | .45 |
| DHA | 25% | 26% | 1.1 | .91 | 21% | 30% | 1.7 | .42 |
| 4-HDHA | 8% | 42% | 8.0 | .015 | 25% | 26% | 1.1 | .91 |
| 10-HDHA | 17% | 33% | 2.6 | .17 | 22% | 30% | 1.6 | .48 |
| 14-HDHA | 17% | 33% | 2.8 | .15 | 25% | 25% | 1.0 | .98 |
| 17-HDHA | 18% | 36% | 2.8 | .15 | 18% | 33% | 2.3 | .24 |
| 19,20-EpDPA | 12% | 39% | 4.8 | .041 | 17% | 35% | 2.6 | .16 |
| Other | ||||||||
| Mead | 21% | 30% | 1.7 | .45 | 25% | 26% | 1.1 | .87 |
Each compound was dichotomized according to median split at each timepoint. Odds ratios and p-values were from logistic regressions adjusted for age and parity.Due to the selection process for inclusion in this substudy, the overall risk of preterm birth is 25%.
9,10-EpOME & 9,10,13-TriHOME had >50% values below LOQ. Concentrations: fatty acids (ug/mL); oxylipins (ng/mL).
Fig. 1.
Concentrations of n-6 fatty acids and oxylipin derivatives in the ORIP plasma substudy (n = 48).*corresponds to p < 0.05 Table 5.
Fig. 2.
Concentrations of n-3 fatty acids and oxylipin derivatives in the ORIP plasma substudy (n = 48). *corresponds to p < 0.05 in Table 5.
None of the oxylipins were predictive of spontaneous preterm birth at pregnancy week 24. However, at 14 weeks of gestation, participants with concentrations of 5-lipoxygenase derivatives of two different precursor fatty acids—5-HETE (OR 8.2; p = 0.014) from AA and 4-HDHA (OR 8.0; p = 0.015) from DHA—above the median had higher risk of spontaneous preterm birth. Participants with concentrations of 15-HETE and 19,20-EpDPA above the median, or 9-HODE below the median, also tended to have higher risk of spontaneous preterm birth (Table 5 and Figs. 1 and 2).
4. Discussion
Preterm birth is a leading cause of infant death and long-term neurological disabilities in children [37,38]. A better understanding of the molecular mechanisms underlying early parturition and the identification of non-invasive mechanism-based biomarkers predicting preterm birth are urgently needed. Supplementation with n-3 DHA during pregnancy has been shown to decrease the risk of preterm birth in singleton pregnancies [19], which may be due, at least in part, to consequent increases in DHA-derived oxylipins with anti-inflammatory and pro-resolving properties, reductions in AA-derived oxylipins that are implicated in immune activation (e.g. leukotrienes, HETEs), or cervical ripening and uterine contractility (e.g. prostanoids), or both. In the present study, DHA supplementation did produce the anticipated increases in unesterified DHA and several DHA-derived oxylipins in plasma. Unexpectedly, however, DHA supplementation did not decrease measured AA-derived oxylipins but rather appeared to increase unesterified AA and one of its oxylipin derivatives (12-HETE). This increase in unesterified AA stands in contrast to the finding that DHA supplementation in the full ORIP cohort decreased total AA in whole blood [23]. Given the small sample size these unexpected findings could be a random error. Alternatively, they may reflect different effects of DHA supplementation on the abundance of AA and AA-derived oxylipins in unesterified versus total lipid pools. Since AA and oxylipins in the unesterified pool are bioactive and could potentially affect early parturition, the effect of supplemental DHA on the unesterified pool of AA and AA-derived oxylipins is a topic that is worthy of investigation in future, larger studies. Concentrations of prostanoids were below the limit of quantitation in all plasma samples, thus the effects of DHA supplementation on plasma prostaglandins could not be tested.
4.1. Unesterified fatty acids as potential biomarkers of spontaneous preterm birth
Unesterified PUFAs and their oxylipin derivatives are attractive candidate biomarkers for predicting risk of spontaneous preterm birth because they may reflect underlying biological mechanisms and are likely to be readily modifiable via diet or supplementation. In the present study, women with AA above the median at 24 weeks of gestation had a higher risk of spontaneous preterm birth than women with AA below the median. Since AA is the precursor to 2-series prostaglandins that promote initiation and progression of labor [9,39] and 5-lipoxygenase derivatives (e.g. leukotriene B4) that are also involved in parturition [3,16,17] in pregnancy, there are plausible biological mechanisms that could account for this finding. However, given the small size of the ORIP plasma subgroup, larger, more adequately powered studies are needed to test the hypothesis that unesterified AA could serve as a mechanism-based biomarker for spontaneous preterm birth.
4.2. Unesterified oxylipins as potential biomarkers of preterm birth
At 14 weeks of gestation, participants with concentrations of 5-lipoxygenase derivatives of AA (5-HETE) and DHA (4-HDHA) above the median had 8-fold higher odds of spontaneous preterm birth compared to participants with concentrations below the median. The 5-lipoxygenase metabolite of AA, leukotriene B4, has previously been shown to be elevated in amniotic fluid during normal parturition [17], and to be elevated in amniotic fluid of mothers with premature rupture of membranes in the presence of infection, labor, or both [3]. In 2016, Maddipati et al. [40] reported that two 5-lipoxygenase derivatives of AA (5-HETE and leukotriene B4) were elevated in amniotic fluid in pregnant women who had intra-amniotic infection, and suggested that these 5-lipoxygenase derivatives could serve as potential biomarkers of microbial invasion of the amniotic cavity, a well-established cause of preterm birth [41]. The above authors did not measure unesterified AA or 5-lipoxygenase derivatives of DHA such as 4-HDHA that was reported in the present study. However, these collective findings in amniotic fluid, together with our findings of higher risk of preterm birth in mothers with higher concentration of 5-HETE and 4-HDHA at 14-weeks, and higher AA concentrations at 24-weeks of gestation, provide an intriguing clue that 5-lipoxygenase derivatives of AA and/or DHA could potentially help identify mothers at risk for spontaneous preterm birth and/or chorioamniotic infection. These preliminary findings should be followed-up in adequately powered studies.
4.3. Oxidized linoleic acid metabolites (OXLAMs) in pregnancy
In the present study LA and unesterified LA-derived oxylipins did not change during pregnancy, were not altered by DHA supplementation, and did not appear to have any relationship with preterm birth. Given the small sample size of this study, these findings do not rule out an effect of OXLAMs or LA in spontaneous preterm birth. Nevertheless, the findings here suggest that follow-up studies emphasizing the relationships between plasma AA, DHA and their respective oxylipin derivatives and spontaneous preterm birth are likely to be more productive. Since the majority of OXLAMs in oxidized lipoproteins are esterified in phospholipids and cholesteryl-esters pools, future studies in this domain should consider quantifying total or esterified OXLAMs rather than the unesterified pool.
5. Limitations
This study had several important limitations. First, although participants were chosen from a randomized trial of DHA supplementation, treatment group was not considered in the selection procedure for this study. Treatment group comparisons may be subject to confounding and the small sample size precludes adequate controls for confounding. Results of comparisons between treatment groups should thus be interpreted with some caution. Additional limitations include the lack of dietary fatty acid data, incomplete understanding of bioavailability and metabolic fate of unesterified fatty acids and small sample sizes. Findings from this substudy are not necessarily generalizable to a more general population of pregnant women. A substantial number of samples had oxylipin values that were below the LOQ. Blood was kept in EDTA tubes at room temperature for up to 2-hours prior to centrifugation and therefore changes in 12-lipoxygenase (12-HETE, 14-HDHA) and platelet derived (TXB2) should be interpreted with some caution in this setting.
6. Summary and conclusion
Here we take an initial step toward understanding the role of oxylipins and their precursor unesterified fatty acids during pregnancy using plasma samples collected from a subset of pregnant Australian women enrolled in the ORIP trial. We showed that DHA supplementation produced marked alterations in unesterified fatty acids and several of their oxylipin derivatives and suggest that unesterified AA and/or 5-lipoxygenase derivatives of AA and DHA hold promise as candidate biomarkers for predicting spontaneous preterm birth.
Supplementary Material
Acknowledgments
Supported by the intramural programs of the National Institute on Alcohol Abuse and Alcoholism and National Institute on Aging, National Institutes of Health, the University of Adelaide, the South Australian Health and Medical Research Institute, and USDA National Institute of Food and Agriculture, Hatch/Taha (project #1008787). The original study was supported by grants from the Australian National Health and Medical Research Council (NHMRC) (1050468) and the Thyne Reid Foundation, and fellowships awarded to M Makrides (1061704), RA Gibson (1046207) and LN Yelland (1052388) from the Australian NHMRC. Croda UK provided the oils and Wassen UK the encapsulation. Collection of the extra samples used in this study were supported by the NHMRC Centre of Research Excellence in Foods for Future Australians (1035530). We would like to acknowledge the ORIP Steering Committee for their contributions to the main trial and Beverly Muhlhausler for assistance with location and transport of samples. We would also like to acknowledge the ORIP study participants.
Declaration of Competing Interest
Dr. Makrides reports receiving advisory board fees, paid to her institution, from Fonterra Co-operative Group and Nestlé Nutrition Institute; and Dr. Gibson, receiving advisory board fees from Fonterra Co-operative Group and holding a patent (WO20 13/10 40 25 A1) on stabilizing and analyzing fatty acids in a biologic sample stored on solid media, owned by Adelaide Research and Innovation, the University of Adelaide, and licensed to Xerion. These disclosures are outside the work reported in this paper.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.plefa.2019.102041.
References
- [1].Yuan ZX, Majchrzak-Hong S, Keyes GS, Iadarola MJ, Mannes AJ, Ramsden CE, Lipidomic profiling of targeted oxylipins with ultra-performance liquid chromatography-tandem mass spectrometry, Anal. Bioanal. Chem. 410 (23) (2018) 6009–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].R Romero, Emamian M, Wan M, Quintero R, Hobbins JC, Mitchel MD, Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor, Am. J. Obstet. Gynecol 157 (6) (1987) 1461–1467. [DOI] [PubMed] [Google Scholar]
- [3].Romero R, Quintero R, Emamian M, Wan M, Grzyboski C, Hobbins JC, et al. , Arachidonate lipoxygenase metabolites in amniotic fluid of women with intra-amniotic infection and preterm labor, Am. J. Obstet. Gynecol 157 (6) (1987) 1454–1460. [DOI] [PubMed] [Google Scholar]
- [4].Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology, Nature 510 (7503) (2014) 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nordgren TM, Berry A Anderson, Van Ormer M, Zoucha S, Elliott E, Johnson R, et al. , Omega-3 fatty acid supplementation, pro-resolving mediators, and clinical outcomes in maternal-infant pairs, Nutrients 11 (1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shearer GC, Newman JW, Impact of circulating esterified eicosanoids and other oxylipins on endothelial function, Curr. Atheroscler. Rep 11 (6) (2009) 403–410. [DOI] [PubMed] [Google Scholar]
- [7].Adili R, Hawley M, Holinstat M, Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids, Prostaglandins Other Lipid Mediat. 139 (2018) 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gouveia-Figueira S, Martens DS, Nawrot TS, Nording ML, Cord blood eicosanoid signatures and newborn gestational age, Prostaglandins Other Lipid Mediat. 133 (2017) 123–127. [DOI] [PubMed] [Google Scholar]
- [9].Olson DM, Ammann C, Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour, Front. Biosci 12 (2007) 1329–1343. [DOI] [PubMed] [Google Scholar]
- [10].Thomas J, Fairclough A, Kavanagh J, Kelly AJ, Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term, Cochrane Database Syst. Rev. (6) (2014) CD003101. [DOI] [PMC free article] [PubMed]
- [11].Boyle AK, Rinaldi SF, Norman JE, Stock SJ, Preterm birth: Inflammation, fetal injury and treatment strategies, J. Reprod. Immunol 119 (2017) 62–66. [DOI] [PubMed] [Google Scholar]
- [12].I Christiaens DB Zaragoza, L Guilbert, SA Robertson, BF Mitchell, DM Olson, Inflammatory processes in preterm and term parturition, J. Reprod. Immunol 79 (1) (2008) 50–57. [DOI] [PubMed] [Google Scholar]
- [13].Reece MS, McGregor JA, Allen KG, Harris MA, Maternal and perinatal long-chain fatty acids: possible roles in preterm birth, Am. J. Obstet. Gynecol 176 (4) (1997) 907–914. [DOI] [PubMed] [Google Scholar]
- [14].Martens DS, Gouveia S, Madhloum N, Janssen BG, Plusquin M, Vanpoucke C, et al. , Neonatal cord blood oxylipins and exposure to particulate matter in the early-life environment: an ENVIR, Environ. Health Perspect 125 (4) (2017) 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bernard JY, Pan H, Aris IM, Moreno-Betancur M, Soh SE, Yap F, et al. , Long-chain polyunsaturated fatty acids, gestation duration, and birth size: a Mendelian randomization study using fatty acid desaturase variants, Am. J. Clin. Nutr 108 (1) (2018) 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].van der Elst CW, Lòpez Bernal A, Sinclair-Smith CC, The role of chorioamnionitis and prostaglandins in preterm labor, Obstet. Gynecol 77 (5) (1991) 672–676. [PubMed] [Google Scholar]
- [17].Romero R, Emamian M, Wan M, Grzyboski C, Hobbins JC, Mitchell MD, Increased concentrations of arachidonic acid lipoxygenase metabolites in amniotic fluid during parturition, Obstet. Gynecol 70 (6) (1987) 849–851. [PubMed] [Google Scholar]
- [18].Kuipers RS, Luxwolda MF, Sango WS, Kwesigabo G, Dijck-Brouwer DA, Muskiet FA, Maternal DHA equilibrium during pregnancy and lactation is reached at an erythrocyte DHA content of 8 g/100 g fatty acids, J. Nutr 141 (3) (2011) 418–427. [DOI] [PubMed] [Google Scholar]
- [19].Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P, et al. , Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial, JAMA 304 (15) (2010) 1675–1683. [DOI] [PubMed] [Google Scholar]
- [20].Makrides M, DHA supplementation during the perinatal period and neurodevelopment: do some babies benefit more than others? Prostaglandins Leukot. Essent. Fatty Acids 88 (1) (2013) 87–90. [DOI] [PubMed] [Google Scholar]
- [21].Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M, Omega-3 fatty acid addition during pregnancy, Cochrane Database Syst. Rev. 11 (2018) CD003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou SJ, Best K, Gibson R, McPhee A, Yelland L, Quinlivan J, et al. , Study protocol for a randomised controlled trial evaluating the effect of prenatal omega-3 LCPUFA supplementation to reduce the incidence of preterm birth: the ORIP trial, BMJ Open 7 (9) (2017) e018360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Makrides M, Best K, Yelland L, McPhee A, Zhou S, Quinlivan J, et al. , A randomized trial of prenatal n-3 fatty acid supplementation and preterm delivery, N. Engl. J. Med 381 (11) (2019) 1035–1045. [DOI] [PubMed] [Google Scholar]
- [24].Foster BA, Escaname E, Powell TL, Larsen B, Siddiqui SK, Menchaca J, et al. , Randomized controlled trial of DHA supplementation during pregnancy: child adiposity outcomes, Nutrients 9 (6) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Olsen SF, Is supplementation with marine omega-3 fatty acids during pregnancy a useful tool in the prevention of preterm birth? Clin Obstet Gynecol. 47 (4) (2004) 768–774 discussion 881–2. [DOI] [PubMed] [Google Scholar]
- [26].Roman AS, Schreher J, Mackenzie AP, Nathanielsz PW, Omega-3 fatty acids and decidual cell prostaglandin production in response to the inflammatory cytokine IL1beta, Am. J. Obstet. Gynecol 195 (6) (2006) 1693–1699. [DOI] [PubMed] [Google Scholar]
- [27].Keelan JA, Mas E, D’Vaz N, Dunstan JA, Li S, Barden AE, et al. , Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors, Reproduction 149 (2) (2015) 171–178. [DOI] [PubMed] [Google Scholar]
- [28].Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, et al. , Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968–73), BMJ 353 (2016) i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dye JF, Jablenska R, Donnell JL, Lawrence L, Leach L, Clark P, et al. , Phenotype of the endothelium in the human term placenta, Placenta 22 (1) (2001) 32–43. [DOI] [PubMed] [Google Scholar]
- [30].T Fournier, Handschuh K, Tsatsaris V, Evain-Brion D, Involvement of PPARgamma in human trophoblast invasion, Placenta 28 (Suppl A) (2007) S76–S81. [DOI] [PubMed] [Google Scholar]
- [31].Palani S, Maksimow M, Miiluniemi M, Auvinen K, Jalkanen S, Salm M, Stabilin-1/CLEVER-1, a type 2 macrophage marker, is an adhesion and scavenging molecule on human placental macrophages, Eur. J. Immunol 41 (7) (2011) 2052–2063. [DOI] [PubMed] [Google Scholar]
- [32].Dubé E, Grave A, Martin C, Desparois G, Moussa I, Ethier-Chiasson M, et al. , Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta, Biol. Reprod 87 (1) (2012) 14 1–1. [DOI] [PubMed] [Google Scholar]
- [33].Choi SH, Sviridov D, Miller YI, Oxidized cholesteryl esters and inflammation, Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862 (4) (2017) 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Indart A, Viana M, Grootveld MC, Silwood CJ, Sanchez-Vera I, Bonet B, Teratogenic actions of thermally-stressed culinary oils in rats, Free Radic. Res. 36 (10) (2002) 1051–1058. [DOI] [PubMed] [Google Scholar]
- [35].Ramsden CE, Hennebelle M, Schuster S, Keyes GS, Johnson CD, Kirpich IA, et al. , Effects of diets enriched in linoleic acid and its peroxidation products on brain fatty acids, oxylipins, and aldehydes in mice, Biochim Biophys. Acta Mol. Cell Biol. Lipids 1863 (10) (2018) 1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stuart EA, Matching methods for causal inference: A review and a look forward, Stat Sci. 25 (1) (2010) 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Keelan JA, Newnham JP, Recent advances in the prevention of preterm birth, F1000Res. 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Govindaswami B, Jegatheesan P, Nudelman M, Narasimhan SR, Prevention of prematurity: advances and opportunities, Clin. Perinatol 45 (3) (2018) 579–595. [DOI] [PubMed] [Google Scholar]
- [39].CD Hsu, Meaddough E, Aversa K, Hong SF, Lee IS, Bahodo-Singh RO, et al. , Dual roles of amniotic fluid nitric oxide and prostaglandin E2 in preterm labor with intra-amniotic infection, Am. J. Perinatol 15 (12) (1998) 683–687. [DOI] [PubMed] [Google Scholar]
- [40].KR Maddipati, Romero R, Chaiworapongsa T, Chaemsaithong P, Zhou SL, Xu Z, et al. , Lipidomic analysis of patients with microbial invasion of the amniotic cavity reveals up-regulation of leukotriene B4, FASEB J. 30 (10) (2016) 3296–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].CC Peng, Chang JH, Lin HY, Cheng PJ, Su BH, Intrauterine inflammation, infection, or both (Triple I): a new concept for chorioamnionitis, Pediatr. Neonatol 59 (3) (2018) 231–237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.