Abstract
A variety of studies show that the s-allele of the serotonin transporter genotype (5-HTT) is related to aggression. However, influences of sex and 5-HTT genotype of both subject and opponent have not received as much attention in aggression research. Using a nonhuman primate model, the present study explores differences in rates of aggression exhibited by 201 group-housed male and female rhesus monkeys (Macaca mulatta; 122 females; 79 males) exposed to an unfamiliar age- and sex-matched stranger while in the presence of other same-sex members of their social group. The study also assesses whether the rates of aggression increase when the home-cage resident, the unfamiliar stimulus animal, or both possess the short (s) allele of the 5-HTT. Results showed that, when compared to females, males exhibited higher rates of physical aggression toward the stranger, and when both the male resident and the male stranger possessed the s-allele, rates of physical aggression toward the stranger increased five-fold. Resident females also engaged in higher rates of physical aggression when they possessed the s-allele, although unlike the males, their physical aggression was directed toward familiar same-sex members of their social group. The findings of this study indicate that rates of physical aggression are modulated by 5-HTT resident and stranger suggest a role of sexual competition in the phenotype of the 5-HTT genotype. Importantly, when two males with impulse deficits, as a function of the s-allele, are placed together, rates of violence exhibited by the dyad escalate substantially.
Keywords: Physical aggression, Sex differences, 5-HTT, Rhesus macaque
1. Introduction
Although studies show that men are more likely to engage in violence than women (Archer, 2004; Bjorkqvist, 2018; Falk et al., 2014; Federal Bureau of Investigation, 2018; Klasios, 2019), women also engage in a sizable proportion of violence (Chesney-Lind and Pasko, 2012), with a recent report indicating that females account for 22% of arrests for physical crimes in the United States (Federal Bureau of Investigation, 2018). Similarly, 17.2% of high school girls engaged in at least one or two incidents of physical aggression in the past year (Centers for Disease Control and Prevention, 2017). The high costs of violence to individuals, communities, and societies make it an important public health issue. As such, identifying risk factors for violence in both sexes an important research aim (Schlomer et al., 2015).
Most research on aggression has focused on males. As a proximal mechanism, prenatal androgens masculinize the male structures of the brain, leading to a number of sex differences in behavior. Maccoby and Jacklin (1978) monograph reports that the largest sex difference seen in early infancy is in aggression, with male infants more aggressive than female infants. A critical review by Archer (2009) focuses on both ultimate and proximal causes of sex difference in aggression. After reviewing relevant research, he concludes that sexual selection is a major reason for sex differences in aggression. Archer also postulates that prenatal androgens are a proximal mechanism for sex differences in aggression, organizing the brain, decreasing impulse control, and leading to impulsive escalated aggression (See also, Higley et al., 1996c). Moreover, prenatal organizational effects sensitize the brain to testosterone, leading to aggression under conditions of provocation later in life.
Evolutionary theory posits that aggression is frequently an adaptive phenomenon, used to defend rank and status in both sexes (Archer, 2009; Campbell, 2013; Hilton et al., 2000; Ness, 2004), and is most often directed toward sexual competitors (Ainsworth and Maner, 2012; Ainsworth and Maner, 2014; Campbell, 1999; Vaillancourt, 2013). As an ultimate mechanism, this is best illustrated in our closest living relatives, the nonhuman primates (hereafter primates). As males of many species of primates reach the age of reproductive capability, they leave their natal troop either because they are driven out by resident males as they attempt consorts and sexual activity with the reproductively able females in the troop, or because young males are motivated by potential access to the reproductively able females in the nearby troop. It is not unusual for migrating males to spend increasing time sitting at the periphery of a new troop, grooming the resident females, ostensibly to facilitate their integration into the new troop (Jack and Pavelka, 1997; Mehlman et al., 1997). Migrating males often suffer intense aggression from the established resident males in the troop that they are attempting to join (Dittus, 1977, 1979). As an ultimate mechanism, such male aggression may result from the established resident males responding to the migrating males as a threat to their rank, resources, and reproductive success (Berard, 1999; MacCormick et al., 2012; Smith, 1993; Wilson and Gordon, 1979). Competition is intense, with one study showing that three-quarters of resident male coalitions are formed to exclude young immigrants (Berghänel et al., 2010). Studies show that a sizable number of these immigrating males are killed by the established male residents while they are attempting to immigrate into the new troop (Dittus, 1977, 1979). Proximal mechanisms that motivate emigration are likely a fear of injury from their natal troop members, and sexual motivation for accessibility to the females in the new group (Drickamer and Vessey, 1973).
In contrast to males, in many species of primates, females remain in their natal troop for life. As an ultimate mechanism, survival of their offspring means that they compete with each other for the limited resources that increase their offsprings’ survival. Judge et al. (1997) studied the effect of crowding on aggression in established troops of rhesus monkey. While male rates of aggression did not vary with increased crowding, rates of female aggression increased significantly, particularly toward unrelated females in the group. Proximally, competition between females typically focuses on access to the limited number of high ranking males because they offer protection to the mother and her infant, and studies show that infants sired by high ranking males grow faster and are more likely to survive (Silk, 2002; Small, 1989). Presumably because of the increased need for nutrition when nursing, access to food and water are central motivators in female competition (Hausfater, 1972; Isbell, 1991; Koenig, 2002; Wrangham, 1981). Unlike resident males who rebuff foreign males, for female macaques, it is not unusual to observe reproductively-able females showing interest in these novel males as reproductive partners (Manson and Perry, 1993). Because high rank provides access to these resources and because females typically remain in the same troop for life, aggression between female primates is most often among familiar females (Cheney et al., 2004; Huchard and Cowlishaw, 2011; Kulik et al., 2015a; Mac-Cormick et al., 2012; Westergaard et al., 2003). This trend in females is also often the case in humans (Campbell, 1986; Campbell, 2013; Hirschinger et al., 2003; Ness, 2004), suggesting phylogenetically old adaptation among primates.
While these sex differences in aggressive behavior are adaptive in nature, issues emerge when aggression escalates to violence. Within both sexes, most violence is committed by only a few individuals (Falk et al., 2014). A number of studies suggest that these individuals may possess deficits in the central serotonin system, which is associated with poor impulse control, including violent behavior (As reviewed by Glick, 2015; Rosell and Siever, 2015; Soubríe, 1986). In both humans (Brown et al., 1979; Coccaro et al., 1996; Virkkunen et al., 1996) and primates (As reviewed by Espinel and Higley, 2013), males with low central serotonin functioning are more likely to engage in physical aggression. While studies on the role of serotonin in aggression in human females yield mixed results (Kuepper et al., 2010a; Manuck et al., 1998; Marsh et al., 2002), which may be due to differences in paradigms and the type of aggression measured, the research on aggression in primate females indicates that the relationship between impaired central serotonin and increased physical aggression is similar to that found in males (Higley et al., 1996a; Westergaard et al., 2003; Westergaard et al., 1999).
Variation in central serotonin functioning is mediated, at least in part, by differences in the functional biallelic serotonin transporter gene (5-HTT), with studies showing environmentally mediated lower central serotonin activity in individuals with the short (s) allele relative to those with the ancestral long (l) allele (Bennett et al., 2002; Reist et al., 2001). 5-HTT genotypic variation is associated with stress-induced cortisol levels (Sorenson et al., 2013) and its association is often in the context of sex and environmental interactions (Barr et al., 2004; Spinelli et al., 2007). Behaviorally, a large number of studies in different human populations indicate that males with the s-allele are more likely to exhibit aggressive behavior and impulsivity (Aluja et al., 2009; Cadoret et al., 2003; Liao et al., 2004; Retz et al., 2004; Verona et al., 2006; Walderhaug et al., 2010; Zimmermann et al., 2009). The relationship between the s-allele and violence is less clear for females, with most studies either not assessing sex differences in the effect of the 5-HTT genotype in mixed-sex samples (Beitchman et al., 2006; Zimmermann et al., 2009) or reporting no effects of 5-HTT genotype on female aggression or impulsivity (Cadoret et al., 2003; Verona et al., 2006; Walderhaug et al., 2010). Some studies, however, report that females with the s-allele are more likely to display displaced aggression when compared to l/l females (Sysoeva et al., 2010), and females with conduct disorder who possess the s-allele are also more likely to engage in aggression (Sakai et al., 2006). Women with the s-allele are also more likely to score high on measures of hostility and self-reported global aggression, when compared to women who are homozygous for the l-allele (Gonda et al., 2009). In one study that directly compared men and women, Conway et al. (2012) found that under conditions of chronic stress, both men and women with the s-allele both showed higher rates of aggression and the strength of the effect was equal for both men and women. This may indicate that like men, the 5-HTT genotype also modulates aggression in women.
Physical aggression often begins as a low-level aggressive response to provocation and escalates into more physical responses (Campbell, 1986; Hamlall and Morrell, 2012; Higley et al., 1996c; Mehlman et al., 1994; Polk, 1999). While individuals with the s-allele may be more likely to respond to provocation with aggression (Peeters et al., 2020; Verona et al., 2006), and the interactions between an aggressor and the individual toward which they are aggressing is likely dynamic, few studies have investigated the genetic characteristics of both partners in an aggressive encounter. As serotonin is produced from tryptophan, Kästner et al. (2019) studied female tryptophan hydroxylase 2 (tph2) knockout mice, pairing them with either wild type or a tph2 companion. As predicted, they found that knockouts exhibited greater aggression. In addition, when both partners were tph2 knockouts the rates of defensive aggression increased considerably. This study suggests that behaviors of two aggressive individuals may create a mutually provocative stimulus that escalates into greater violence. In this current study, the genotypic characteristics of both individuals in an aggressive encounter is for the first time studied in a primate, a partner A-genotype-by-partner B-genotype interaction.
Like humans, rhesus monkeys (Macaca mulatta) possess a functional biallelic orthologue of the 5-HTT genotype after 5-HTT (Bennett et al., 2002; Lesch et al., 1997; Wendland et al., 2006). Rhesus monkeys are particularly aggressive by nature, when compared to most other macaques (Demaria and Thierry, 2001; Sussman et al., 2013; Wendland et al., 2006), and as a consequence they provide an ideal model for the study of aggression. Moreover, studies utilizing rhesus monkeys provide enhanced capacity for experimental control over what initiates aggression and the setting for the response. Rhesus monkeys are territorial, and defend their territory with aggression (Southwick et al., 1974). One standardized test, the Intruder Challenge (Fairbanks, 2001) capitalizes on this by exposing individuals to an unfamiliar age- and sex-matched stranger. In this paradigm, three age and sex-matched subjects from the same social group are tested together. An earlier study showed that rhesus monkeys exhibit a vigorous defensive response in this paradigm that is, in part, mediated by genetic and social background (Schwandt et al., 2010), particularly in males that were reared without their mothers. Specifically, this study showed that subjects with the s-allele and reared in conditions of social deprivation engaged in higher rates of physical aggression, when compared to l/l individuals. As this earlier study focused on the effects of rearing and 5-HTT genotype, it was underpowered to directly compare males and females in the context of resident and stranger 5-HTT genotypes, and the 5-HTT genotype of the stranger was not considered for its potential contribution to these outcomes. It was also limited because it did not assess sex differences in aggression between the three males or three females that were tested together. This is relevant because studies show that females are more likely to target familiar females with aggression (Bernstein and Ehardt, 1986); whereas, males are more likely to target unfamiliar males attempting to enter the group (Lindburg, 1971). 5-HTT genotype effects on aggression have also been studied in an undisturbed home-cage setting with the results showing that rates of aggression were greater in subjects with the s-allele but only when they were reared without parents in peer-only (PR) groups, although the sample size was again too small to assess male-female differences (Barr et al., 2003). The present study aims to assesses sex differences in aggression as mediated by the 5-HTT genotype, and does so in the context of the genotype of both the resident and the stranger.
For rhesus monkey males, an emigrating male (i.e., a stranger) represents a risk to a male resident’s reproductive success. It was hypothesized, therefore, that males would exhibit more aggression toward an unfamiliar same-sex stranger, when compared to females. Females, on the other hand do not migrate, and familiar females within their troop are typically the major source of competition for access to the limited resources for reproductive success. As such, it was hypothesized that females would exhibit more aggression toward the familiar females in their group, when compared to males from the same group. As a proximal mechanism, the presence of the s-allele was predicted to amplify the intensity and rates of these natural tendencies, increasing rates and intensity of aggression that males exhibited toward the unfamiliar stimulus animal, and that females exhibited toward their familiar same-sex social partners. Moreover, when both the resident and stranger possess the s-allele, it was hypothesized that aggression would be more frequent and likely to escalate to violence, and that this trend would be exhibited primarily by males.
2. Materials and methods
2.1. Subjects
Subjects were 201 socially housed rhesus macaques (122 females, 79 males; mean age: 50.24 ± 26.86 months) and 19 age-, size-, and sex-matched unfamiliar stimulus animals, from 13 birth cohorts, maintained by the National Institute of Child Health and Development (NICHD) at the National Institutes of Health Animal Center (NIHAC) in Poolesville, MD. All resident monkeys were in stable social groups and had been living together for over a year. To maintain genetic diversity in the colony, matings were planned and male breeders were regularly purchased and transferred to different enclosures after a tenure of three years. This resulted in an overall degree of relatedness (identity-by-descent) of 1.68% (about the level of third-degree cousins), which has been cited as a satisfactory criterion for the assumption of unrelatedness in previous human (Robin et al., 1998) and primate (Newman et al., 2005) studies. Subjects were raised in one of three rearing conditions: mother-reared (MR, n = 94), peer-reared (PR, n = 41), or surrogate/peer-reared (SPR, n = 66). Specific details about rearing conditions can be found elsewhere (Shannon et al., 1998). Briefly, all MR animals were raised in large indoor/outdoor runs in social groups with 8–10 adult females, their offspring, and two adult males. This roughly approximates rhesus macaque social structures in the wild (Lindburg, 1971), albeit on a smaller scale. All PR and SPR residents were separated from their mothers at birth and hand-reared in a neonatal nursery. At 37 days of age, PR infants were rehoused with other age-matched peers, with whom they were in continuous 24/7 contact. The SPR residents remained with their cloth surrogate mother, with two-hour daily social interactions with same-aged peers in a playroom, five days a week. All residents, including MR residents, remained in these conditions until approximately seven months of life, when they were integrated into larger social groups of 20 to 30 mixed peers. Just prior to adolescence, males and females were divided into smaller, same-sex groups where the females remained until the present study. Adult males were typically removed from the colony and replaced by outside males, which were introduced into the previously all-female groups. The living quarters for the social groups where the residents lived were large indoor-outdoor runs (indoor: 2.44 × 3.05 × 2.21 m; outdoor: 2.44 × 3.0 × 2.44 m), with lighting in inside of the pens maintained on a 12:12 cycle (0700–1900). Residents had ad libitum access to water and were fed Purina® High Protein Monkey Chow (#5038), with fresh fruit provided three times weekly and assorted seeds provided daily. Animal care followed the standards listed by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1985) and all protocols for the use of experimental animals were approved by the Institutional Animal Care and Use Committee of the National Institute of Alcohol Abuse and Alcoholism.
2.2. Intruder challenge test
Using a cross sectional design, the subjects were tested over the course of one year, except for the youngest group that was tested a few months later. The Intruder Challenge test is used to measure individual differences in temperament including aggression and impulsivity (Fairbanks, 2001) in a controlled manner where the risk of serious injury to the residents or unfamiliar stimulus animal is minimized. In brief, three residents from the same group were tested in their home-cage for their response to an unfamiliar stimulus animal that was placed outside the larger outdoor portion of the cage in which they lived.
2.2.1. Unfamiliar stimulus animal
The stranger was age-, sex-, and size-matched to residents that were being tested. The strangers were randomly selected from other social groups that were outside of visual or auditory contact of the residents’ living quarters. None of the strangers had any previous interactions with the residents. Just before testing, each unfamiliar stimulus animal was removed from its social group, placed into a smaller holding cage (0.76 m × 0.63 m 0.91 m), and isolated in a room some distance from the testing area where it was acclimated to the smaller holding cage for a 30-min period before testing began. Each of the unfamiliar stimulus animals were used in 3–5 sessions to assure consistency of the stranger’s behavior and analyses showed no differences in the residents’ behavior for the order in which they were tested with the stranger (first second, etc.). The stranger’s holding cage had an open mesh front and top so that the stranger was visible to the resident test animals, but the holding cage was large enough to allow the stranger to retreat to the back of the cage where it was out of reach of the residents. None of the strangers were tested as residents.
2.2.2. Test animal residents
Each session of the Intruder Challenge test assessed three same-age, same-sex residents, and each resident was only tested once. There were a total of 54 triads, with one subject dropped from the analyses due to genotype uncertainty. The residents in the trio were randomly selected from their larger social group, and placed together into the outdoor portion of their home enclosure (2.44 × 3.0 × 2.44 m) for a 10-min acclimation period. Formal testing began when the stranger’s cage was placed immediately next to the front of the outdoor portion of the focal resident’s home enclosure so that the focal resident could approach the stranger and interact through the mesh of the cage. Based on the methods designed by Fairbanks and Jorgensen (2011) and our own pilot testing, the residents’ outdoor run and the stranger’s holding cage were placed so that the two fronts touched, with mesh preventing full body contact to avoid injury to any of the animals. The size of the mesh allowed the stranger and residents to place their hands through the mesh to make contact with the other animal, but the mesh was small enough to avoid biting the face or body of the residents or the stranger. None of the subjects or strangers were wounded or injured during testing, with contact aggression consisting mainly of swipes, hand slaps, and grabs.
2.3. Behavior coding
As the behaviors of three residents were recorded in one session, one observer/rater was assigned to behaviorally code each resident’s behaviors toward the stranger, as well as toward the other group members (three raters total) each test. Behaviors were recorded using handheld computers equipped with Observer® software (Noldus, Leesburg, Virginia). The software allows each observer to record the rates and duration of various behaviors performed or received by the test resident. Using an exhaustive and mutually-exclusive ethogram that has been utilized in an earlier study (Schwandt et al., 2010), residents were observed for 30 min using continuous recording, with the targets and rates of competitive and physical aggression recorded by the observers (See Tables 1 and 2). While no observer was assigned to the stranger, aggressive behaviors directed by the stranger toward a resident were scored by that resident’s observer. Measures of impulsivity and social behaviors in which the residents engaged were also recorded—latency and frequency to approach the stranger, as well as time spent in social contact with the stranger or familiar, same-sex cagemates, or in grooming with their cagemates (See Tables 1 and 2). Interrater behavior coding reliability was established at r > 0.85, and raters were blind to 5-HTT genotype status for both residents and strangers. (See Figs. 1 and 2.)
Table 1.
Behaviors involving the stranger.
| Behavior Observed | Description |
|---|---|
| Resident Directs Competitive Aggression at Stranger* | Aggression without physical contact (e.g., open-mouth threats, lunges, and barks) directed by the resident toward the stranger |
| Resident Directs Physical Aggression at Stranger* | Aggression involving physical contact, including bites, slaps, grabs, and swipes directed by the resident toward the stranger |
| Stranger Directs Competitive Aggression at Resident* | Aggression without physical contact (e.g., open-mouth threats, lunges) directed by the stranger toward the resident |
| Stranger Directs Physical Aggression at Resident* | Aggression involving physical contact, including bites and slaps, directed by the stranger toward the resident |
| Latency of Resident to approach Stranger | The time (in seconds) from the beginning of the test until the test resident first approaches within 1 m of the stranger animal (as indicated by a measured circular marking on the floor of the test enclosure). |
| Resident’s Frequency of Approaching the Stranger* | Number of times during the 30-min test that the test resident approaches within 1 m of the stranger animal |
| Resident’ Social Contact with Stranger | Sitting, standing, lying, or engaging in locomotion within arm’s reach (in reference to the resident) of the stranger |
Note.
Denotes behaviors that were scored as frequencies (rates/per 30 min session). Behaviors without asterisks were scored as duration (seconds/per 30 min session).
Table 2.
Behaviors involving other same-sex residents.
| Behavior Observed | Description |
|---|---|
| Resident Directs Competitive Aggression at Other Same-Sex Residents* | Aggression without physical contact (e.g., open-mouth threats, lunges) directed by the resident toward other same-sex residents |
| Resident Directs Physical Aggression at Other Same-Sex Residents* | Aggression involving physical contact, including bites and slaps, directed by the resident toward other same-sex residents |
| Other Same-Sex Residents Directs Competitive Aggression at Resident* | Aggression without physical contact (e.g., open-mouth threats, lunges) directed by the other same-sex residents toward the resident |
| Other Same-Sex Residents Directs Physical Aggression at Resident* | Aggression involving physical contact, including bites and slaps, directed toward the resident by other same-sex residents |
| Social Contact with Other Same-Sex Residents | Resident engages in sitting, standing, lying, or engaging in locomotion within arm’s reach (in reference to the resident) of the other same-sex residents. |
| Give Groom to Other Same-Sex Residents | Resident runs fingers or mouth through the pelage of other same-sex residents. Also includes scratching, biting, licking, or rubbing. |
Note.
Denotes behaviors that were scored as frequencies (rates/per 30 min session). Behaviors without asterisks were scored as durations (seconds/per 30 min session).
Fig. 1. Effect of Resident Sex and Resident Genotype on Physical Aggression Directed at the Stranger.
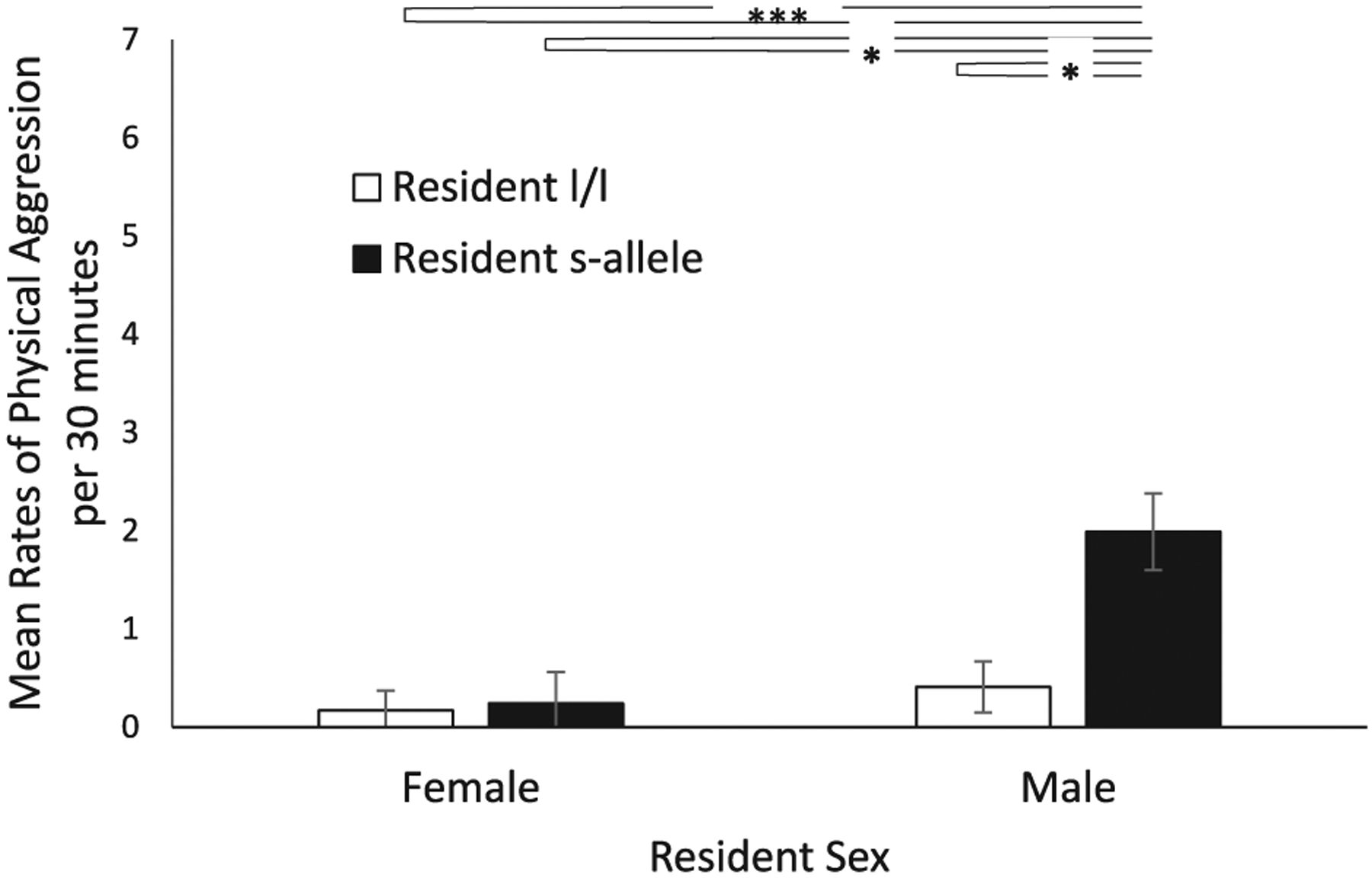
There was a significant sex-by-resident-genotype interaction p = .001), with male residents that possessed the s-allele engaging in higher rates of physical aggression toward the stranger when compared to male residents with the l/l genotype or female residents with either genotype. Note that the data represent the response of the residents without considering the genotype of the stranger—see Fig. 2 for this consideration. Data are presented as mean rates per 30 min. White bars indicate l/l genotype residents and black bars indicate s-allele genotype residents. * denotes p < .05, **** denotes p < .001. Error bars are standard errors.
Fig. 2. Effect of Resident Genotype and Intruder Genotype on Physical Aggression Directed at the Stranger.
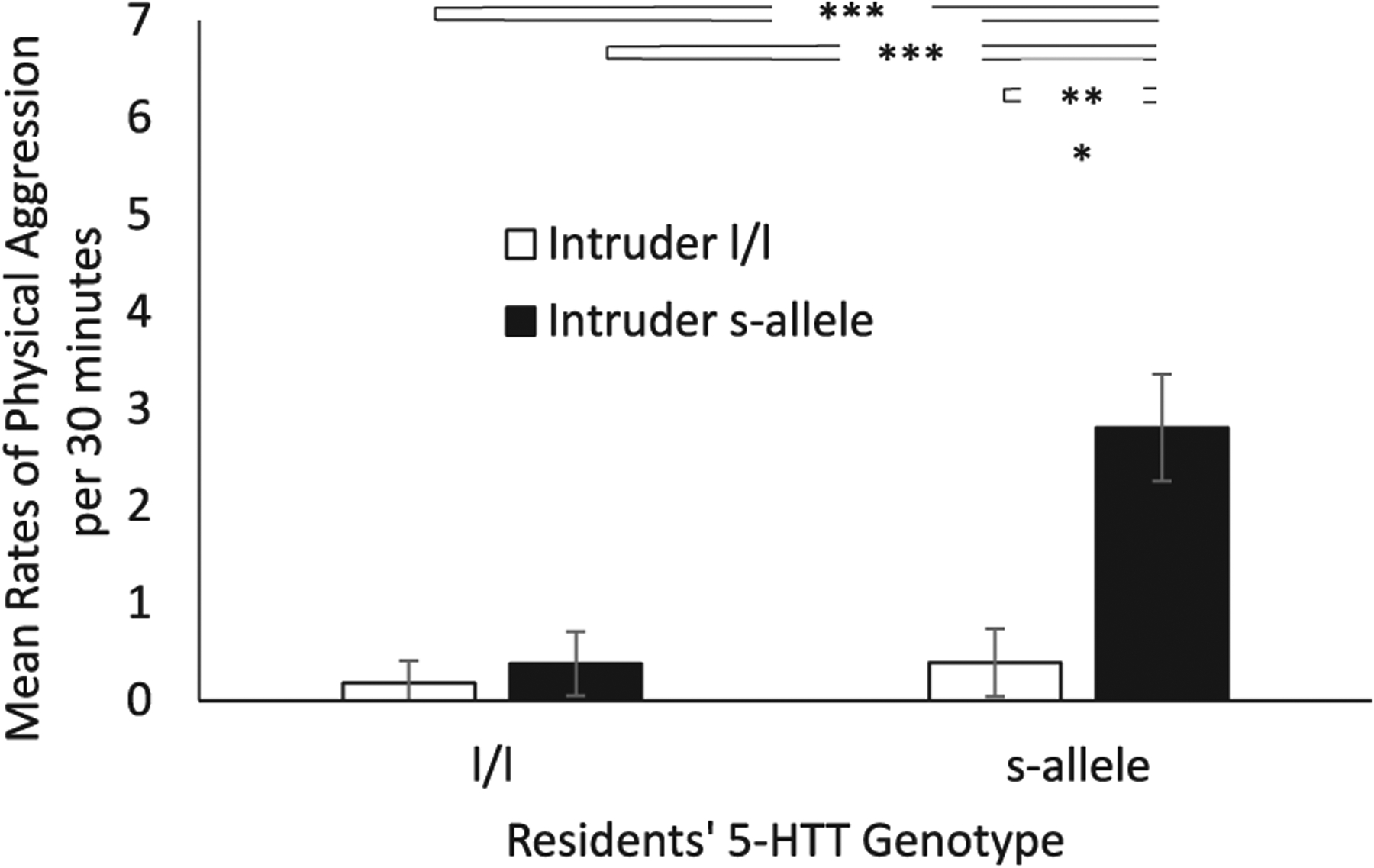
There was a significant resident-genotype-by-stranger-genotype interaction (p = .002), with physical aggression toward the stranger escalating above that of all other groupings when both the resident and stranger possessed the s-allele. Data are presented as mean rates per 30 min. Means are reported as rates per 30 min. White bars indicate l/l strangers and black bars indicate s-allele strangers. **** denotes p < .001. Error bars are standard errors.
2.4. Genotyping
5-HTT genotyping methods have been described in detail elsewhere (Barr et al., 2004). According to standard extraction methods, DNA was isolated from whole blood, collected during routine health checks in infancy for the majority of residents (except for males purchased as breeders, which had blood collected in adulthood). The 5-HTT was amplified from 25 ng of genomic DNA with flanking oligonucleotide primers (stpr5, 5-GGCGTTGCCGCTCTGAATGC; intl, 5′- CAGGGGA-GATCCTGGGAGGG) in 15-μL reactions with Platinum™ Taq and the PCRX Enhancer System kit, according to the manufacturer’s protocol (Invitrogen™, Carlsbad, California). Amplifications were performed on a Perkin Elmer ® (Wellesley, Massachusetts) thermocycler (9700) with one cycle at 96 °C for 5 min followed by 30 cycles of 94 °C for 15 s, 60 °C for 15 s, 72 °C for 30 s, and a final 3-min extension at 72 °C. Amplicons were separated by electrophoresis on a 10% polyacrylamide gel, and the short (s, 388 bp) and long (l, 419 bp) alleles of the rh5-HTT were identified by direct visualization after ethidium bromide staining.
2.5. Data analyses
For analyses examining aggression directed toward the stranger by residents or by the stranger toward the residents, a series of three-way analyses of variance (ANOVAs) were conducted, with resident 5-HTT genotype, stranger 5-HTT genotype, and the sex of the dyad as independent variables, and the rates of competitive or physical aggression directed toward the stranger by residents or by the stranger toward residents as the dependent variable. For analyses examining the resident’s aggressive and non-aggressive interactions with other same-sex residents, as well as non-aggressive behaviors of residents toward the stranger, two-way ANOVAs were used, with resident genotype and sex as the independent variables and behaviors with other residents or the stranger as the dependent variables (see Tables 1 and 2). All data are reported as mean frequency or duration. Effect sizes for main effects and interactions in the ANOVA model were calculated using partial η2 (ηp2), with effect sizes being interpreted as 0.02 as a small effect, 0.13 a medium effect, and 0.26 as a large effect. Effect sizes for pairwise comparisons were reported using Cohen’s d, with an effect size of 0.2 considered a small effect, 0.5 as a moderate effect, and 0.8 and greater as a large effect (Cohen, 1988). Differences were considered significant at p < .05.
As previous studies (Higley et al., 1996a; Kulik et al., 2015a) show that age affects aggressive behavior in rhesus macaques, test age of the residents was included as a covariate in all analyses. Because the last group was tested a few months later, we performed a one-way ANOVA comparing the last group tested with the subjects tested in the same year and found no difference (p > .60). We also divided the subjects into those tested in the first half of the study and those tested in the second half of the study. One-way analyses showed no difference in subjects tested early in the year and those tested later in the year (p > .58), an indication that the procedures and behavioral responses did not drift across the months of study. Preliminary analyses suggested no difference in variables of interest between SPR and PR residents (p > .05); therefore, SPR and PR residents were combined into one category. Further analyses showed no effect of rearing on rates of any of the types of aggression assessed in this study (p > .05). Nevertheless, because other studies show that rearing condition interacts with 5-HTT genotype (Barr et al., 2003; Reif et al., 2007; Schwandt et al., 2010; Wood et al., 2021), rearing condition was controlled for in the analyses. Due to the low number (n = 3) of residents that were homozygous for the s-allele, the s/s residents were combined with the l/s genotype residents for the purposes of the analysis, as has been done in previous studies (Bennett et al., 2002; Champoux et al., 2002). Genotype frequencies did not deviate from Hardy-Weinberg equilibrium. See Table 3 for sex and genotype characteristics of residents. All data were analyzed using SPSS 26 (IBM, 2019). Results are reported as mean rates or duration in seconds /per 30 min session and standard errors of the mean. As the data are part of a larger database that is shared, we note the data of the current study are available on request.
Table 3.
Sex and genotype distributions of residents.
| 5-HTT Genotype | l/l | s-allele | Total | |
|---|---|---|---|---|
| Sex | Female | 87 | 35 | 122 |
| Male | 56 | 23 | 79 |
3. Results
3.1. Competitive aggression directed by residents toward the stranger
There was a main effect of sex for competitive aggression directed by residents toward the stranger (F(1,151) = 12.01, p < .001, ηp2 = 0.07), with male residents directing more frequent competitive aggression toward the stranger when compared to females (Males: Mn = 2.58 ± 0.52; Females: Mn = 0.23 ± 0.43; d = 0.56). No other main effects or interactions attained statistical significance for competitive aggression exhibited toward the stranger.
3.2. Physical aggression directed by residents toward the stranger
There was a statistically significant main effect of sex (F(1,195) = 10.36, p = .002; ηp2 = 0.05), with male residents engaging in more physical aggression toward the stranger, when compared to female residents (Males: Mn = 1.20 ± 0.24; Females: 0.21 ± 0.19; d = 0.47). There was also a main effect of resident genotype (F(1,195) = 7.48, p = .007; ηp2 = 0.04), with residents that possessed the s-allele engaging in higher rates of physical aggression toward the stranger (Mn = 1.11 ± 0.25, p = .007; d = 0.49), when compared to l/l residents (Mn: 0.29 ± 0.16; d = 0.49).
There was a two-way sex-by-resident-genotype interaction (F (1,195) = 6.49, p = .01; ηp2 = 0.03—see Fig. 1). Further analyses showed that the rates of physical aggression toward the stranger were higher in resident males with the s-allele (Mn = 3.14 ± 0.44, p < .001; d = 0.83), when compared to resident males with the l/l genotype (Mn = 0.65 ± 0.31) and female residents with the l/l genotype (Mn = 0.08 ± 0.23) and females with the s-allele (Mn = 0.31 ± 0.38).
There was also a significant two-way resident-genotype-by-stranger-genotype interaction (F(1,193) = 7.64, p < .001; ηp2 = 0.07—See Fig. 2). Rates of physical aggression by the resident toward the stranger were highest when the resident and the stranger both possessed the s-allele (Mn = 3.04 ± 0.49, p < .001; d = 1.28), when compared to dyads where both the resident and intruder had the l/l genotype (Mn = 0.59 ± 0.32). None of the other comparisons were statistically significant (p > .05).
There was a similar two-way resident sex-by-stranger-genotype interaction, (F(1,151) = 22.30 p < .001, ηp2 = 0.13) on physical aggression toward the stranger, with subsequent analyses showing that male residents directed higher rates of physical aggression toward strangers with the s-allele (Mn: 3.46 ± 0.44, p < .001), when compared to male (Mn: 0.32 ± 0.32) or female residents directing physical aggression toward strangers with the l/l genotype (Mn: 0.13 ± 0.37). There were no other significant differences between any of the other comparisons (p > .05).
There was also a main effect of stranger genotype (F(1,155) = 10.98, p = .001, ηp2 = 0.07), with the higher rates of aggression occurring when the stranger possessed the s-allele (Mn: 1.39 ± 0.28, p = .001; d = 0.57) relative to when the stranger was homozygous for the l-allele (Mn: 0.25 ± 0.19). Perhaps of most interest was the significant three-way resident-sex-by-resident-genotype-by-stranger-genotype interaction (F(1, 151) = 9.28, p = .003, ηp2 = 0.06—see Fig. 3; see Table 4). When both the male residents and male strangers possessed the s-allele, they exhibited rates of physical aggression toward the stranger that were significantly higher than any other sex and 5-HTT genotype combination (Mn: 5.80 ± 0.70, p < .001; d ≥ 2.53), escalating in rate to nearly three times more physical aggression than when the male residents’ genotype was not considered (see Fig. 1). There were no other significant differences when the other groupings were compared (p > .05). Female residents rarely exhibited physical aggression toward the female stranger, regardless of their genotype or that of the female stranger (see Table 4 & Fig. 4.)
Fig. 3.
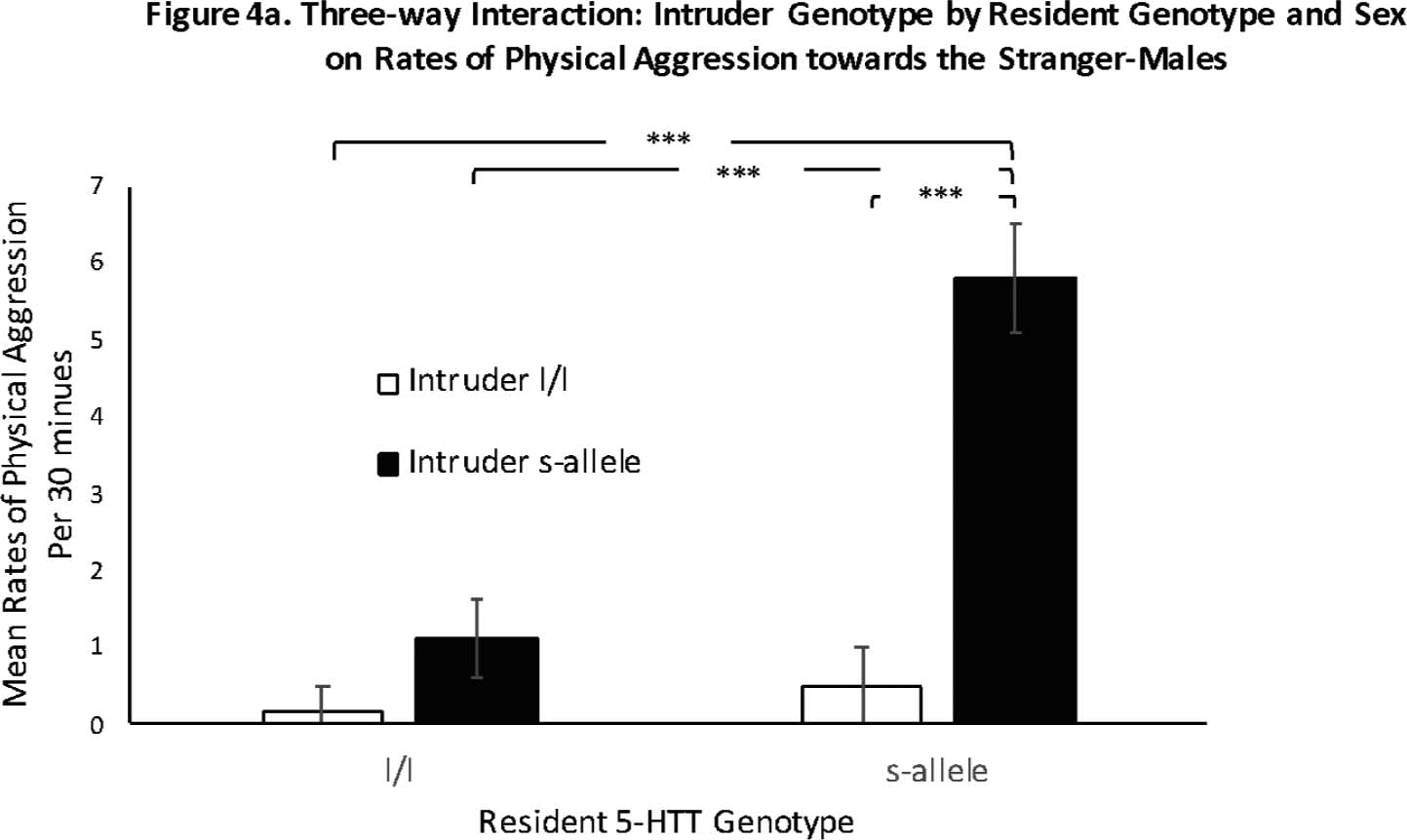
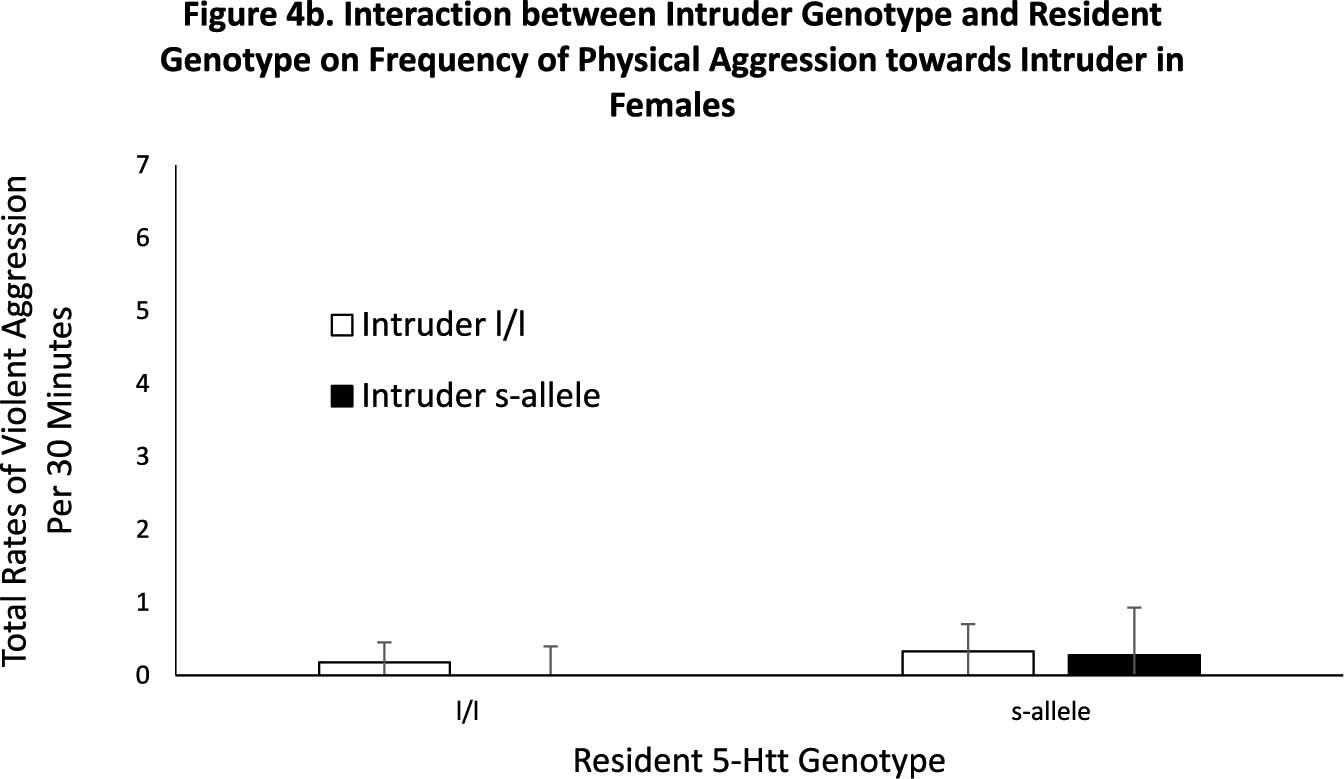
Figure 3a. Three-way Interaction: Intruder Genotype by Resident Genotype and Sex on Rates of Physical Aggression towards the Stranger-Males
Figure 3b. Interaction between Intruder Genotype and Resident Genotype on Frequency of Physical Aggression towards Intruder in Females
Fig. 3a and b. There was a significant three-way, resident sex-by-resident-genotype interaction (p = .001), with male residents that possessed the s-allele engaging in higher rates of physical aggression against the stranger than did l/l male residents or female residents of either genotype. Note that the data show the response of the residents in the context of the genotype of the stranger—see Fig. 2. Female residents rarely exhibited physical aggression toward the stranger, regardless of genotype. Data are presented as mean rates per 30 min. White bars indicate l/l genotype residents and black bars indicate s-allele genotype residents. * denotes p < .05, **** denotes p < .001. Error bars are standard errors.
Table 4.
Means and SE of physical aggression directed by resident toward stranger.
| Stranger 5-HTT × Resident 5-HTT | ||||
|---|---|---|---|---|
| sex | Stranger: l/l, Resident: l/l | Stranger: l/l, Resident: s-allele | Stranger: s-allele, Resident: l/l | Stranger: s-allele, Resident: s-allele |
| Females | 0.18 ± 0.28 | 0.33 ± 0.40 | 0.00 ± 0.38 | 0.28 ± 0.65 |
| Males | 0.17 ± 0.33 | 0.48 ± 0.53 | 1.12 ± 0.51 | 5.80 ± 0.70 |
Fig. 4. Effect of Resident Sex and Resident Genotype on Physical Aggression towards Familiar Cage-Mates.
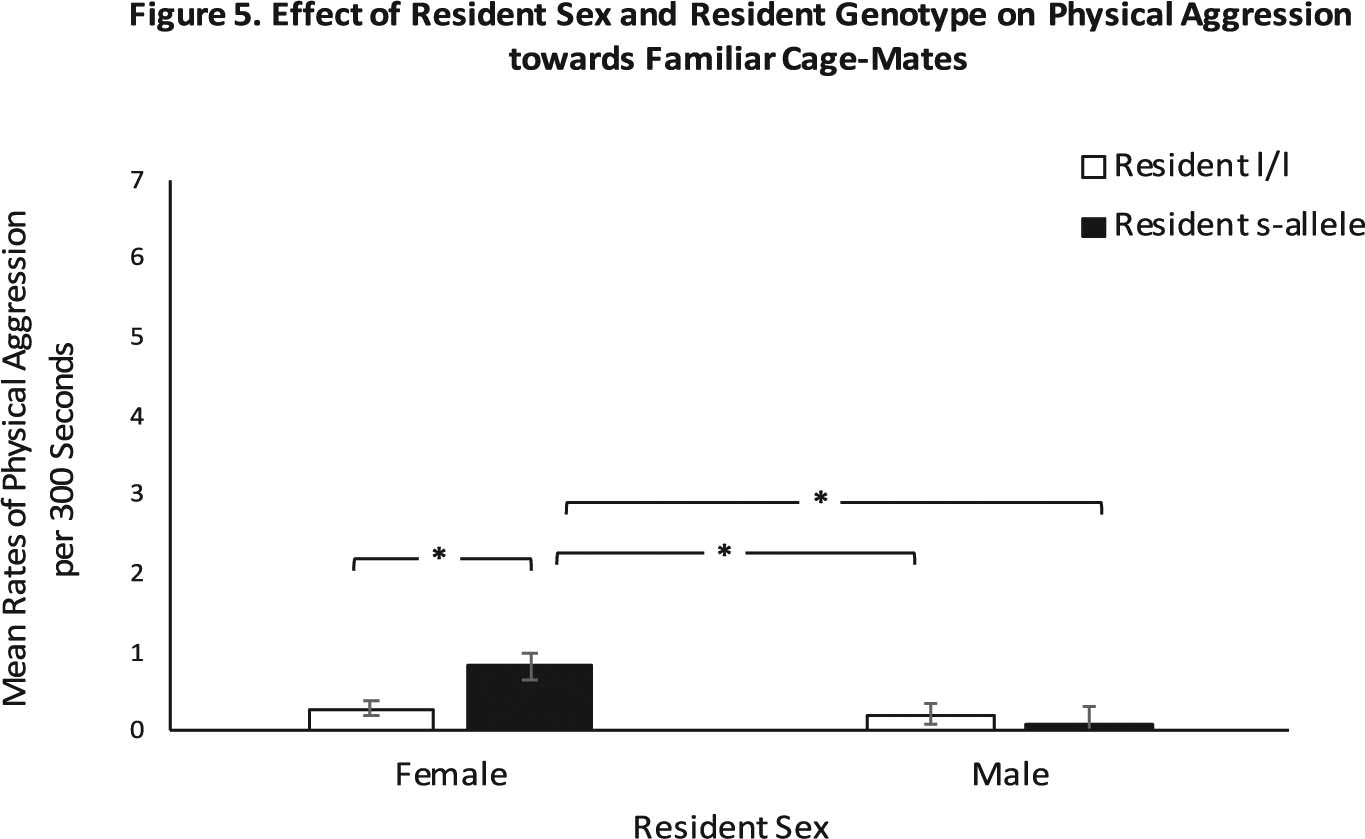
Shows the sex-by-resident-genotype interaction (p = .03), with the resident females that possessed the s-allele directing more physical aggression toward the other two familiar same-sex residents compared to the l/l genotype females or males of either genotype. White bars indicate l/l residents and black bars indicate s-allele residents. * denotes p < .05. Error bars are standard errors.
3.3. Residents’ competitive aggression directed at the other same-sex residents
There were no statistically significant main effects or interactions for competitive aggression directed by residents toward other familiar same-sex residents (p > .05).
3.4. Residents’ physical aggression directed at the other same-sex residents
Analyses indicated a main effect of sex for physical aggression by the resident directed toward the other residents (F(1,195) = 6.35, p = .01; ηp2 = 0.03), with the female residents directing higher rates of physical aggression toward the other familiar home-cage residents (Mn: 0.55 ± 0.10, p = .01; d = 0.37), when compared to male residents (Mn: 0.15 ± 0.12). Resident genotype did not exert a statistically significant main effect on this behavior (F(1,195) = 1.80, p = .18, ηp2 = 0.01). There was, however, a statistically significant sex-by-resident-genotype interaction (F(1,195) = 4.72, p = .03, ηp2 = 0.02), with the female residents with the s-allele directing more physical aggression toward the other two familiar same-sex residents (Mn: 0.83 ± 0.17, p = .01; d = 0.48), when compared to their l/l female counterparts (Mn: 0.28 ± 0.11), or males of either genotype (l/l: Mn: 0.21 ± 0.13; s-allele: Mn: 0.09 ± 0.21). Resident males seldom showed physical aggression against each other (See Fig. 4).
3.5. Competitive aggression from same-sex residents directed at the residents
There were no significant effects for competitive aggression by other same-sex residents directed at the other residents (p > .05).
3.6. Physical aggression from same-sex residents directed at the residents
There was a statistically significant main effect regarding physical aggression between the three familiar, same-sex residents, although there was a marginally significant main effect for sex (F(1,195) = 3.40, p = .07, ηp2 = 0.02), with the female same-sex residents directing more frequent physical aggression at each other (Mn: 0.38 ± 0.08, p = .07; d = 0.27), when compared to the male residents (Mn: 0.15 ± 0.10). There was also a trending effect of resident genotype (F(1, 195) = 2.97, p = .09, ηp2 = 0.02). Specifically, when the resident possessed the s-allele they received more physical aggression from the other two same-sex residents (Mn: 0.37 ± 0.10, p = .09; d = 0.27), compared to their l/l peers (Mn: 0.16 ± 0.07).
3.7. Competitive aggression directed by stranger toward the residents
Results from analyses indicated a statistically significant main effect of sex on competitive aggression directed by the stranger toward the residents (F(1,195) = 12.25, p < .001, ηp2 = 0.06), with male strangers exhibiting higher rates of competitive aggression toward the resident (Mn: 0.75 ± 0.15, p < .0001; d = 0.45), when compared to female strangers (Mn: 0.07 ± 0.12). There were no other main effects or interactions for competitive aggression directed by the stranger toward the residents (p > .05).
3.8. Physical aggression directed by the stranger toward the residents
There was a statistically significant main effect of sex on physical aggression directed by the stranger toward the residents (F(1,151) = 20.14, p < .001, ηp2 = 0.12), with male strangers directing more frequent rates of physical aggression at the residents (Mn: 1.85 ± 0.29, p < .001; d = 0.73), when compared to female strangers (Mn: 0.14 ± 0.24). There was also a main effect of resident genotype on the strangers’ rate of physical aggression directed at residents (F(1,151) = 16.74, p < .001, ηp2 = 0.10), with strangers directing more frequent physical aggression at residents with the s-allele (Mn: 1.77 ± 0.32, p < .001; d = 0.71), when compared to rates of physical aggression directed toward residents with the l/l genotype (Mn: 0.22 ± 0.21). Similarly, there was a main effect of stranger genotype on the strangers’ rate of aggression directed at residents (F(1,151) = 18.33, p < .001, ηp2 = 0.11), with strangers with the s-allele directing more frequent physical aggression toward the resident (Mn: 1.80 ± 0.31, p < .001; d = 0.73), than did strangers with the l/l genotype (Mn: 0.19 ± 0.21).
There was a two-way resident-genotype-by-stranger-genotype interaction (F(1,151) = 16.68, p < .001, ηp2 = 0.10), with strangers possessing the s-allele directing more physical aggression toward the s-allele residents (Mn: 0.48 ± 0.15, p < .001, d ≥ 1.51), than any other pairing of genotypes (resident l/l with stranger s-allele: Mn: 0.18 ± 0.14; resident l/l with stranger l/l: Mn: 0.17 ± 0.09; resident s-allele with stranger l/l: Mn: 0.33 ± 0.22). There were no significant differences in other resident-genotype-by-stranger-genotype comparisons (p > .05). There was also a two-way sex-by-resident-genotype interaction for physical aggression (F(1,151) = 18.61, p < .001, ηp2 = 0.11), with strangers directing higher rates of physical aggression toward male residents with the s-allele (Mn: 3.42 ± 0.48, p < .001; d = 1.34), when compared to male residents with the l/l genotype (Mn: 0.27 ± 0.33), and female residents of either genotype (l/l: Mn: 0.17 ± 0.25; s-allele: Mn: 0.12 ± 0.41). There were no significant differences between any other sex-by-resident-genotype comparisons (p > .05). There was also a two-way sex-by-stranger-genotype interaction (F(1,151) = 16.40, p < .001, ηp2 = 0.10), with male strangers with the s-allele exhibiting higher rates of aggression toward residents (Mn: 3.41 ± 0.47, p < .001; d ≥ 1.44), when compared to any other sex-by-stranger-genotype pairing. There were no significant differences between any other sex-by-stranger-genotype comparisons (p > .05).
Finally, there was a statistically significant three-way sex-by-resident-genotype-by-stranger-genotype interaction (F(1,151) = 14.24, p < .001, ηp2 = 0.09; see Table 5). Further analyses showed that when the stranger and resident males both possessed the s-allele, strangers with the s-allele exhibited significantly higher rates of physical aggression than any other combination (Mn: 6.45 ± 0.76, p < .001, d ≥ 3.05; see Table 5). There were no other comparisons that attained statistical significance (p > .05).
Table 5.
Means and SE of Physical Aggression Directed by Stranger toward Resident.
| Stranger 5-HTT × Resident 5-HTT | ||||
|---|---|---|---|---|
| sex | Stranger: l/l, Resident: l/l | Stranger: l/l, Resident: s-allele | Stranger: s-allele, Resident: l/l | Stranger: s-allele, Resident: s-allele |
| Females | 0.18 ± 0.30 | 0.00 ± 0.43 | 0.15 ± 0.40 | 0.23 ± 0.70 |
| Males | 0.18 ± 0.36 | 0.39 ± 0.57 | 0.36 ± 0.55 | 6.45 ± 0.76 |
3.9. Resident’s non-aggressive behaviors with the stranger
There was a significant main effect of sex on the latency of residents to initially approach the stranger (F(1,175) = 3.97, p = .048, ηp2 = 0.02), with male residents taking less time to approach the stranger than female residents (males: Mn:200.81 ± 49.30 s; females: Mn: 330.27 ± 40.85 s, p = .048; d = 0.31). There was also a main effect of sex on the amount of time residents spent in close social proximity with the stranger (F(1, 195) = 12.02, p = .001, ηp2 = 0.06), with resident males spending more time in close social proximity to the stranger than resident females (males: Mn:96.15 ± 13.36 s; females: Mn: 35.78 ± 10.71 s, p = .001; d = 0.51). No other main effects, or sex-by-resident-genotype effects for non-aggressive behaviors with the stranger attained statistical significance.
3.10. Other behaviors of residents toward same-sex residents
There was a main effect of sex on the amount of time that residents spent in close proximity with the other two same-sex residents, (F(1, 194) = 3.97, p = .048, ηp2 = 0.02), with females spending more time in social contact with the other members of their social group, when compared to males (females: Mn:588.41 ± 37.25 s; males: Mn: 467.56 ± 46.64 s, p = .048; d = 0.30). There was also a main effect of sex on time spent grooming the other two members of the group, (F(1, 195) = 4.42, p = .04, ηp2 = 0.02), with females spending more time grooming the other two members of their group than did males (females: Mn:12.51 ± 3.34 s; males: Mn: 1.09 ± 4.16 s, p = .04; d = 0.31). There were no other effects of resident genotype on these affiliative behaviors (p > .05).
4. Discussion
In support of the hypotheses of the current study, male rhesus monkeys engaged in higher rates of physical aggression toward the unfamiliar stranger, when compared to female rhesus monkeys. Also consistent with the study’s hypotheses, resident females exhibited higher rates of physical aggression and social behaviors toward the familiar same-sex members of their group rather than toward the female stranger. In both sexes, physical aggression behaviors were modulated by 5-HTT genotype, although the target of the physical aggression differed in males and females. For males, the s-allele increased rates of physical aggression against the stranger, and when both the resident male and stranger possessed the s-allele, rates of physical aggression increased more than five-fold, when compared to when the resident alone had the s-allele. Likewise, females with the s-allele engaged in higher rates of physical aggression against their familiar female companions when compared to their l/l female counterparts. It is worth noting that the presence of the s-allele was only associated with physical aggression (bites, slaps, grabs), and not with competitive aggression (milder aggression, used to maintain status-threats barks, stares). This finding is consistent with other studies showing that impaired CNS serotonin, as measured by CSF 5-HIAA concentrations, is correlated with physical aggression, but not with competitive forms of aggression (Higley et al., 1996b).
The results of the present study are consistent with other work showing that males engage in higher rates of physical aggression, when compared to females (Archer, 2004, 2009). While somewhat speculative, one possible ultimate explanation for the sex differences in the target of aggressive behavior may be the result of different selective pressures that males and females experienced during their evolutionary histories. Many studies of male Old World primates indicate that high ranking males have more opportunities to breed with females and sire the majority of the offspring in their group (Cowlishaw and Dunbar, 1991; Massen et al., 2012; Paul et al., 1993; Smith, 1993; Witt et al., 1981; Wroblewski et al., 2009). Viewed from a more proximal lens, in wild rhesus monkey troops, an outsider male attempting to join a new troop represents a threat to the rank. Similarly, studies in humans show that most violence between young males occurs over threats to status (Ainsworth and Maner, 2012; Wilson and Daly, 1985) and most violence between males is directed at strangers (Hilton et al., 2000; Morgan and Truman, 2020). Consistent with the findings of Schwandt et al. (2010), males in the current study largely targeted their aggression against the unfamiliar male stranger. Males also exhibited more nonaggressive behaviors focused at the stranger than did females. This may reflect an increased tendency to gain proximity to the stranger in order to act aggressively. Males showed a shorter latency to approach the stranger, suggesting that impulsivity played a role. An earlier study in rhesus monkeys showed that levels of impulsivity, as measured by impulsive, dangerous leaps between trees, is positively correlated with rates of physical aggression (Higley et al., 1996b).
Central to the primary hypotheses is the finding that males’ rates of physical aggression escalated when both the resident and stranger possessed the s-allele, which to the authors’ knowledge is the first demonstration of a resident-5-HTT-by-stranger-5-HTT genotype interaction in an aggressive encounter. That males’ rates of physical aggression were highest, compared to females, may be related to the organizational effects of prenatal androgen exposure (Archer, 2009), which may lead to decreased impulse control. Other studies suggest that the s-allele of the 5-HTT genotype is associated with impulsivity and violent behavior (Lesch et al., 1996). Given other work indicating that combinations of naturally-occurring, trait-like combinations of high testosterone and decreased serotonin availability in healthy men, but not women (Kuepper et al., 2010b), it is possible that the high rates of physical aggression between males with the s-allele in this study is, in part, mediated by inherent testosterone and serotonin availability.
The stressful setting in which the aggression occurred may be related to the following explanation. Provocation is a prominent cue for impending aggressive behavior (Bettencourt and Miller, 1996; Campbell, 1986; Hamlall and Morrell, 2012; Mazefsky and Farrell, 2005; Polk, 1999), particularly among those prone to act aggressively (Higley et al., 1996b; Mehlman et al., 1994; Peeters et al., 2020; Verona et al., 2006). As such, one interpretation of the spiraling rate of physical aggression exhibited when both partners possessed the s-allele is that the reciprocal provocation between two aggressive partners led to rates of violence that rapidly escalated. Such findings are reminiscent of descriptions of road rage where, as Harding et al. (1998) note, it is often difficult to decouple the perpetrator from the victim, especially when both participants use their automobiles in an aggressive manner. In Parsons (1978) review of the causes of road rage, the author also indicates that individuals involved in incidents of road rage were disproportionately likely to have been involved in previous incidents of violence and that provocation triggered the rage, suggesting a trait-like response to challenge. A study of prison homicides among male inmates in Texas (USA) by Cunningham et al. (2010) found that the majority of prison homicides (54.3%) started with an argument or altercation that escalated after the initial provocation, a finding consistent with the results of this study. The findings of this study align well with an interpretation that, in a provocative situation, including the provocation of another impulsive individual, males with the s-allele, are more likely to exhibit unrestrained and aggressive behavior.
An earlier study, utilizing this same paradigm (Schwandt et al., 2010), assessed the relationship between early social deprivation and 5-HTT genotype on physical aggression toward the stranger, showing that socially-deprived males with the s-allele exhibit higher rates of aggression. This study built, in part, upon those results, compared the rates of aggression among males and females with the s-allele during an aggressive encounter. Perhaps one of the most interesting findings of the current study is that like males, females with the s-allele exhibited increased rates of aggression, but this physical aggression was directed toward familiar same-sex cage-mates, rather than the stranger. Due to rhesus macaque social biology, an unfamiliar female migrating to the new group is an unlikely event, and as such, a response to a novel female would not be the likely source of selection in females of this species. However, from an ultimate perspective, females may be selected to demonstrate aggression toward other females in their social group, which are competitors for reproductive opportunities and resources. This, however, does not explain why the presence of a female stranger led the residents to react physically toward other females of the group. One possibility is that the stimulus of a novel female may have increased arousal and emotionality, leading to physical response toward same-sex residents. Another possibility is that, with nowhere to escape, the aggression becomes redirected toward the other females who are acting similarly. A more likely proximal explanation is that the aggression toward the other two resident females may represent a challenge to social status. As female rank is a product of social support and coalitions, by randomly selecting the three females to be tested each day, females likely, in at least some cases, lost their coalitionary social support; aggression to the other two females may represent a challenge to the status quo in social status. In this explanation, the unfamiliar stimulus female is not so much eliciting aggression, but instead, the aggression is a result of the loss of social support from the other females from their social group. Female residents also demonstrated more affiliative social behaviors with other females in their group than did males. While this could reflect the females’ maintaining close proximity to act aggressively, increased social affiliation between females is consistent with other studies showing that females are more likely to show social affiliation than are males (Cords et al., 2010; Kulik et al., 2015b), and they are more likely than males to reconcile after aggression, as measured by increased social proximity, and, in particular, time spent in social grooming following conflicts (Cooper et al., 2005). While the sequence of behaviors is unknown in the Stranger Challenge paradigm, it is possible that the grooming and social affiliation represent reconciling with their familiar same-sex residents as a consequence of aggression. Paralleling the findings from this study, in humans, the targets of most female-female aggression are unrelated sexual rivals (Arnocky et al., 2012; Burbank, 1987; Vaillancourt, 2013). While these forms of aggression are often indirect, they can quickly escalate to violence, especially when desirable males are in short supply (Campbell, 2004; Ness, 2004).
This study further bolsters Archer (2009) evolutionary explanation for sex differences in rates of physical aggression and is likely to have implications for the study of human aggression. Consistent with principles of sexual competition, sex differences in aggression were most frequently focused at potential sexual rivals in both males and females. Moreover, these natural tendencies were amplified and exaggerated by the presence of the s-allele in both sexes. Previous studies show that aggression is most likely under conditions of provocation, particularly in subjects prone to impulsive aggression (Higley et al., 1992; Higley et al., 1996c). As a proximal mechanism, the Intruder Paradigm is specifically designed to elicit aggression. Studies show that subjects with the s-allele are more prone to impulsivity and aggression, and it is likely that the provocation of the Intruder Paradigm setting interacts with genotype, leading to increased aggression in the subjects with the s-allele. Given the many deleterious outcomes of impulsive behavior and excessive aggression, previous research has investigated why the s-allele has been maintained in the rhesus monkey population, and suggested that males with the s-allele may be able to reproduce earlier by entering a new troop at a younger age (Trefilov et al., 2000). The current study offers further explanation for the preservation of the s-allele in rhesus monkey populations by suggesting that differences in 5-HTT phenotype may exert a balancing effect by enabling males with the s-allele to prevent reproductive rivals from entering the troop, while allowing females with the s-allele to outcompete sexual rivals from within the troop.
5. Conclusions
The current study is the first of its kind to examine the role of sex, resident genotype, and partner genotype in aggressive encounters. Most importantly, the study suggests that a better understanding of aggression comes when sex and characteristics of both individuals in an aggressive encounter are considered. Identifying and understanding evolutionary-based risk factors for physical crime and harm to others is essential to developing effective preventative measures in modern societies. Research investigating sex-by-genotype interactions and, in particular, the contributions of genotype in the perpetrator and target may reveal several valuable novel findings to aggression research in the years to come.
Acknowledgements
This study was supported by the Intramural Research Programs of the National Institute on Alcohol Abuse and Alcoholism, and the National Institute of Child Health and Human Development, and graduate funding from Brigham Young University. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Child Health and Human Development, or Brigham Young University.
The authors would like to thank the research and animal care staff, as well as the graduate students and post-docs at the National Institutes of Health. We would also like to thank the undergraduates from Brigham Young University.
Footnotes
Declaration of Competing Interest
None.
Data availability
As the data are part of a larger database that is not owned by the primary authors, we note in the manuscript that the data are available on request.
References
- Ainsworth SE, Maner JK, 2012. Sex begets violence: mating motives, social dominance, and physical aggression in men. J. Pers. Soc. Psychol 103, 819–829. [DOI] [PubMed] [Google Scholar]
- Ainsworth SE, Maner JK, 2014. Assailing the competition: sexual selection, proximate mating motives, and aggressive behavior in men. Pers. Soc. Psychol. B 40, 1648–1658. [DOI] [PubMed] [Google Scholar]
- Aluja A, Garcia LF, Blanch A, De Lorenzo D, Fibla J, 2009. Impulsive-disinhibited personality and serotonin transporter gene polymorphisms: association study in an inmate’s sample. J. Psychiatr. Res 43, 906–914. [DOI] [PubMed] [Google Scholar]
- Archer J, 2004. Sex differences in aggression in real-world settings: a meta-analytic review. Rev. Gen. Psychol 8, 291–322. [Google Scholar]
- Archer J, 2009. Does sexual selection explain human sex differences in aggression? Behav. Brain Sci 32, 249–266. [DOI] [PubMed] [Google Scholar]
- Arnocky S, Sunderani S, Miller JL, Vaillancourt T, 2012. Jealousy mediates the relationship between attractiveness comparison and females’ indirect aggression. Pers. Relationship 19, 290–303. [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD, 2003. The utility of the non-human primate model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2, 336–340. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD, 2004. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol. Psychiatry 55, 733–738. [DOI] [PubMed] [Google Scholar]
- Beitchman JH, Baldassarra L, Mik H, De Luca V, King N, Bender D, Ehtesham S, Kennedy JL, 2006. Serotonin transporter polymorphisms and persistent, pervasive childhood aggression. Am. J. Psychiatry 163, 1103–1105. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD, 2002. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol. Psychiatry 7, 118–122. [DOI] [PubMed] [Google Scholar]
- Berard J, 1999. A four-year study of the association between male dominance rank, residency status, and reproductive activity in rhesus macaques (Macaca mulatta). Primates 40, 159–175. [DOI] [PubMed] [Google Scholar]
- Berghänel A, Schülke O, Ostner J, 2010. Coalition formation among Barbary macaque males: the influence of scramble competition. Anim. Behav 80, 675–682. [Google Scholar]
- Bernstein IS, Ehardt C, 1986. The influence of kinship and socialization on aggressive behaviour in rhesus monkeys (Macaca mulatta). Anim. Behav 34, 739–747. [Google Scholar]
- Bettencourt B, Miller N, 1996. Gender differences in aggression as a function of provocation: a meta-analysis. Psychol. Bull 119, 422–447. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K, 2018. Gender differences in aggression. Curr. Opin. Psychol 19, 39–42. [DOI] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF, 1979. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1, 131–139. [DOI] [PubMed] [Google Scholar]
- Burbank VK, 1987. Female aggression in cross-cultural perspective. Behav. Sci. Res 21, 70–100. [Google Scholar]
- Cadoret RJ, Langbehn D, Caspers K, Troughton EP, Yucuis R, Sandhu HK, Philibert R, 2003. Associations of the serotonin transporter promoter polymorphism with aggressivity, attention deficit, and conduct disorder in an adoptee population. Compr. Psychiatry 44, 88–101. [DOI] [PubMed] [Google Scholar]
- Campbell A, 1986. Self-report of fighting by females: a preliminary study. Br. J. Criminol 26, 28–46. [Google Scholar]
- Campbell A, 1999. Staying alive: evolution, culture, and women’s intrasexual aggression. Behav. Brain Sci 22, 203–214 discussion 214–252. [DOI] [PubMed] [Google Scholar]
- Campbell A, 2004. Female competition: causes, constraints, content, and contexts. J. Sex Res 41, 16–26. [DOI] [PubMed] [Google Scholar]
- Campbell A, 2013. The evolutionary psychology of women’s aggression. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 368, 20130078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2017. High School YRBS.
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ, 2002. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol. Psychiatry 7, 1058–1063. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM, Fischer J, Beehner J, Bergman T, Johnson S, Kitchen DM, Palombit R, Rendall D, Silk JB, 2004. Factors affecting reproduction and mortality among baboons in the Okavango DeltaBotswana. Int. J. Primatol 25, 401–428. [Google Scholar]
- Chesney-Lind M, Pasko L, 2012. The female offender: girls, women, and crime. Sage Publications. [Google Scholar]
- Coccaro EF, Berman ME, Kavoussi RJ, Hauger RL, 1996. Relationship of prolactin response to d-fenfluramine to behavioral and questionnaire assessments of aggression in personality-disordered men. Biol. Psychiatry 40, 157–164. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. In: The Effect Size, Statistical Power Analysis for the Behavioral Sciences, 2nd ed. L. Erlbaum Associates, Hillsdale, N.J., pp. 77–83 [Google Scholar]
- Conway CC, Keenan-Miller D, Hammen C, Lind PA, Najman JM, Brennan PA, 2012. Coaction of stress and serotonin transporter genotype in predicting aggression at the transition to adulthood. J. Clin. Child Adolesc. Psychol 41, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Berntein IS, Hemelrijk CK, 2005. Reconciliation and relationship quality in assamese macaques (Macaca assamensis). Am. J. Primatol 65, 269–282. [DOI] [PubMed] [Google Scholar]
- Cords M, Sheehan MJ, Ekernas LS, 2010. Sex and age differences in juvenile social priorities in female philopatric, nondespotic blue monkeys. Am. J. Primatol 72, 193–205. [DOI] [PubMed] [Google Scholar]
- Cowlishaw G, Dunbar RI, 1991. Dominance rank and mating success in male primates. Anim. Behav 41, 1045–1056. [Google Scholar]
- Cunningham MD, Sorensen JR, Vigen MP, Woods S, 2010. Inmate homicides: killers, victims, motives, and circumstances. J. Crim Justice 38, 348–358. [Google Scholar]
- Demaria C, Thierry B, 2001. A comparative study of reconciliation in rhesus and tonkean macaques. Behaviour 138, 397–410. [Google Scholar]
- Dittus WP, 1977. The social regulation of population density and age-sex distribution in the toque monkey. Behaviour 63, 281–322. [Google Scholar]
- Dittus WP, 1979. The evolution of behaviors regulating density and age-specific sex ratios in a primate population. Behaviour 69, 265–301. [Google Scholar]
- Drickamer LC, Vessey SH, 1973. Group changing in free-ranging male rhesus monkeys. Primates 14, 359–368. [Google Scholar]
- Espinel WF, Higley JD, 2013. A nonhuman primate model of serotonin-mediated violence and antisocial behavior—a decade and-a-half update. In: Hall FS (Ed.), Serotonin: Biosynthesis, Regulation and Health Implications. Nova Science Publishers, Inc, pp. 69–96. [Google Scholar]
- Fairbanks LA, 2001. Individual differences in response to a stranger: social impulsivity as a dimension of temperament in vervet monkeys (Cercopithecus aethiops sabaeus). J. Comp. Psychol 115, 22–28. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Jorgensen MJ, 2011. Objective behavioral tests of temperament in nonhuman primates. In: Personality and Temperament in Nonhuman Primates. Springer, pp. 103–127. [Google Scholar]
- Falk Ö, Wallinius M, Lundström S, Frisell T, Anckarsäter H, Kerekes N, 2014. The 1% of the population accountable for 63% of all violent crime convictions. Soc. Psychiatry Psychiatr. Epidemiol 49, 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Bureau of Investigation, 2018. Crime in the U.S. 2018.
- Glick AR, 2015. The role of serotonin in impulsive aggression, suicide, and homicide in adolescents and adults: a literature review. Int. J. Adolesc. Med. Health 27, 143–150. [DOI] [PubMed] [Google Scholar]
- Gonda X, Fountoulakis KN, Juhasz G, Rihmer Z, Lazary J, Laszik A, Akiskal HS, Bagdy G, 2009. Association of the s allele of the 5-HTTLPR with neuroticism-related traits and temperaments in a psychiatrically healthy population. Eur. Arch. Psychiatry Clin. Neurosci 259, 106–113. [DOI] [PubMed] [Google Scholar]
- Hamlall V, Morrell R, 2012. Conflict, provocation and fights among boys in a south african high school. Gend. Educ 24, 483–498. [Google Scholar]
- Harding R, Morgan F, Indermaur D, Ferrante A, Blagg H, 1998. Road rage and the epidemiology of violence: something old, something new. STAYSAFE 58, 202–219. [Google Scholar]
- Hausfater G, 1972. Intergroup behavior of free-ranging rhesus monkeys (Macaca mulatta). Folia Primatol. 18, 78–107. [DOI] [PubMed] [Google Scholar]
- Health, N.I.O., 1985. Guide for the Care and Use of Laboratory Animals. National Academies. [Google Scholar]
- Higley JD, Mehlman P, Taub D, Higley S, Suomi SJ, Linnoila M, Vickers J, 1992. Cerebrospinal fluid monoamine and adrenal correlates of aggression in free-ranging rhesus monkeys. Arch. Gen. Psychiatry 49, 436–441. [DOI] [PubMed] [Google Scholar]
- Higley JD, King ST Jr., Hasert MF, Champoux M, Suomi SJ, Linnoila M, 1996a. Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology 14, 67–76. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Higley SB, Fernald B, Vickers J, Lindell SG, Taub DM, Suomi SJ, Linnoila M, 1996b. Excessive mortality in young free-ranging male nonhuman primates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Arch. Gen. Psychiatry 53, 537–543. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M, 1996c. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol. Psychiatry 40, 1067–1082. [DOI] [PubMed] [Google Scholar]
- Hilton NZ, Harris GT, Rice ME, 2000. The functions of aggression by male teenagers. J. Pers. Soc. Psychol 79, 988–994. [DOI] [PubMed] [Google Scholar]
- Hirschinger NB, Grisso JA, Wallace DB, McCollum KF, Schwarz DF, Sammel MD, Brensinger C, Anderson E, 2003. A case-control study of female-to-female nonintimate violence in an urban area. Am. J. Public Health 93, 1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchard E, Cowlishaw G, 2011. Female–female aggression around mating: an extra cost of sociality in a multimale primate society. Behav. Ecol 22, 1003–1011. [Google Scholar]
- IBM Corp. Released, 2019. IBM SPSS Statistics for Windows. Version 26.0. IBM Corp, Armonk, NY. [Google Scholar]
- Isbell LA, 1991. Contest and scramble competition: patterns of female aggression and ranging behavior among primates. Behav. Ecol 2, 143–155. [Google Scholar]
- Jack KM, Pavelka M, 1997. The behavior of peripheral males during the mating season in Macaca fuscata. Primates 38, 369–377. [Google Scholar]
- Judge PG, Waal, De Frans, B.M., 1997. Rhesus monkey behaviour under diverse population densities: Coping with long-term crowding. Animal Behaviour 54 (3), 643–662. 10.1006/anbe.1997.0469. [DOI] [PubMed] [Google Scholar]
- Kästner N, Richter SH, Urbanik S, Kunert J, Waider J, Lesch K-P, Kaiser S, Sachser N, 2019. Brain serotonin deficiency affects female aggression. Sci. Rep 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasios J, 2019. Aggression among men: an integrated evolutionary explanation. Aggr. Violent Behav 47, 29–45. [Google Scholar]
- Koenig A, 2002. Competition for resources and its behavioral consequences among female primates. Int. J. Primatol 23, 759–783. [Google Scholar]
- Kuepper Y, Alexander N, Osinsky R, Mueller E, Schmitz A, Netter P, Hennig J, 2010a. Aggression–interactions of serotonin and testosterone in healthy men and women. Behav. Brain Res 206, 93–100. [DOI] [PubMed] [Google Scholar]
- Kuepper Y, Alexander N, Osinsky R, Mueller E, Schmitz A, Netter P, Hennig J, 2010b. Aggression—interactions of serotonin and testosterone in healthy men and women. Behav. Brain Res 206, 93–100. [DOI] [PubMed] [Google Scholar]
- Kulik L, Amici F, Langos D, Widdig A, 2015a. Sex differences in the development of aggressive behavior in rhesus macaques (Macaca mulatta). Int. J. Primatol 36, 764–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik L, Amici F, Langos D, Widdig A, 2015b. Sex differences in the development of social relationships in rhesus macaques (Macaca mulatta). Int. J. Primatol 36, 353–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL, 1996. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A, 1997. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. J. Neural Transm 104, 1259–1266. [DOI] [PubMed] [Google Scholar]
- Liao D-L, Hong C-J, Shih H-L, Tsai S-J, 2004. Possible association between serotonin transporter promoter region polymorphism and extremely violent crime in chinese males. Neuropsychobiology 50, 284–287. [DOI] [PubMed] [Google Scholar]
- Lindburg DG, 1971. The rhesus monkey in North India: An ecological and behavioral study. In: Rosenblum LA (Ed.), Primate Behavior: Developments in Field and Laboratory Research, pp. 1–106. [Google Scholar]
- Maccoby EE, Jacklin CN, 1978. The psychology of sex differences. Stanford University Press. [Google Scholar]
- MacCormick HA, MacNulty DR, Bosacker AL, Lehman C, Bailey A, Anthony Collins D, Packer C, 2012. Male and female aggression: lessons from sex, rank, age, and injury in olive baboons. Behav. Ecol 23, 684–691. [Google Scholar]
- Manson JH, Perry SE, 1993. Inbreeding avoidance in rhesus macaques: whose choice? Am. J. Phys. Anthropol 90, 335–344. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF, 1998. Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology 19, 287–299. [DOI] [PubMed] [Google Scholar]
- Marsh DM, Dougherty DM, Moeller FG, Swann AC, Spiga R, 2002. Laboratory-measured aggressive behavior of women: acute tryptophan depletion and augmentation. Neuropsychopharmacology 26, 660–671. [DOI] [PubMed] [Google Scholar]
- Massen JJ, Overduin-de Vries AM, de Vos-Rouweler AJ, Spruijt BM, Doxiadis GG, Sterck EH, 2012. Male mating tactics in captive rhesus macaques (Macaca mulatta): the influence of dominance, markets, and relationship quality. Int. J. Primatol 33, 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky CA, Farrell AD, 2005. The role of witnessing violence, peer provocation, family support, and parenting practices in the aggressive behavior of rural adolescents. J. Child Fam. Stud 14, 71–85. [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M, 1994. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am. J. Psychiatry 151, 1485–1491. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Fernald BJ, Sallee FR, Suomi SJ, Linnoila M, 1997. CSF 5-HIAA, testosterone, and sociosexual behaviors in free-ranging male rhesus macaques in the mating season. Psychiatry Res. 72, 89–102. [DOI] [PubMed] [Google Scholar]
- Morgan RE, Truman JL, 2020. In: US Department of Justice, O.o.J.P. (Ed.), Criminal Victimization, 2019. Washington, D.C. [Google Scholar]
- Ness CD, 2004. Why girls fight: female youth violence in the inner city. Ann. Am. Acad. Political Soc. Sci 595, 32–48. [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP, 2005. Monoamine oxidase a gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol. Psychiatry 57, 167–172. [DOI] [PubMed] [Google Scholar]
- Parsons KR, 1978. Violence on the Road: A Logical Extension to the Subculture of Violence Thesis? Research Section, Department of Justice. [Google Scholar]
- Paul A, Kuester J, Timme A, Arnemann J, 1993. The association between rank, mating effort, and reproductive success in male Barbary macaques (Macaca sylvanus). Primates 34, 491–502. [Google Scholar]
- Peeters DG, Lange W-G, Von Borries AKL, Franke B, Volman I, Homberg JR, Verkes R-J, Roelofs K, 2020. Threat-avoidance tendencies moderate the link between serotonin transporter genetic variation and reactive aggression. Front. Behav. Neurosci 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk K, 1999. Males and honor contest violence. Homicide Stud. 3, 6–29. [Google Scholar]
- Reif A, Rösler M, Freitag CM, Schneider M, Eujen A, Kissling C, Wenzler D, Jacob CP, Retz-Junginger P, Thome J, 2007. Nature and nurture predispose to violent behavior: serotonergic genes and adverse childhood environment. Neuropsychopharmacology 32, 2375–2383. [DOI] [PubMed] [Google Scholar]
- Reist C, Mazzanti C, Vu R, Tran D, Goldman D, 2001. Serotonin transporter promoter polymorphism is associated with attenuated prolactin response to fenfluramine. Am. J. Med. Genet 105, 363–368. [DOI] [PubMed] [Google Scholar]
- Retz W, Retz-Junginger P, Supprian T, Thome J, Rosler M, 2004. Association of serotonin transporter promoter gene polymorphism with violence: relation with personality disorders, impulsivity, and childhood ADHD psychopathology. Behav. Sci. Law 22, 415–425. [DOI] [PubMed] [Google Scholar]
- Robin RW, Long JC, Rasmussen JK, Albaugh B, Goldman D, 1998. Relationship of binge drinking to alcohol dependence, other psychiatric disorders, and behavioral problems in an american indian tribe. Alcohol.: clinExp. Res 22, 518–523. [PubMed] [Google Scholar]
- Rosell DR, Siever LJ, 2015. The neurobiology of aggression and violence. CNS Spectr. 20, 254–279. [DOI] [PubMed] [Google Scholar]
- Sakai JT, Young SE, Stallings MC, Timberlake D, Smolen A, Stetler GL, Crowley TJ, 2006. Case-control and within-family tests for an association between conduct disorder and 5HTTLPR. Am. J. Med. Genet 141, 825–832. [DOI] [PubMed] [Google Scholar]
- Schlomer GL, Cleveland HH, Vandenbergh DJ, Feinberg ME, Neiderhiser JM, Greenberg MT, Spoth R, Redmond C, 2015. Developmental differences in early adolescent aggression: a gene× environment× intervention analysis. J. Youth Adolesc 44, 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Sjöberg RL, Chisholm KL, Higley JD, Suomi SJ, Heilig M, Barr CS, 2010. Gene–environment interactions and response to social intrusion in male and female rhesus macaques. Biol. Psychiatry 67, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ, 1998. Rearing condition and plasma cortisol in rhesus monkey infants. Am. J. Primatol 46, 311–321. [DOI] [PubMed] [Google Scholar]
- Silk JB, 2002. Kin selection in primate groups. Int. J. Primatol 23, 849–875. [Google Scholar]
- Small MF, 1989. Female choice in nonhuman primates. Am. J. Phys. Anthropol 32, 103–127. [Google Scholar]
- Smith DG, 1993. A 15-year study of the association between dominance rank and reproductive success of male rhesus macaques. Primates 34, 471–480. [Google Scholar]
- Sorenson AN, Sullivan EC, Mendoza SP, Capitanio JP, Higley JD, 2013. Serotonin transporter genotype modulates HPA axis output during stress: effect of stress, dexamethasone test and ACTH challenge. Transl. Dev. Psychiatry 1, 21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubrié P, 1986. Reconciling the role of central serotonin neurons in human and animal behavior. Behav. Brain Sci 9, 319–335. [Google Scholar]
- Southwick CH, Siddiqi M, Farooqui M, Pal B, 1974. Xenophobia among free-ranging rhesus groups in India. In: Holloway RL (Ed.), Primate Aggression, Territoriality, and Xenophobia. Academic Press, Inc, New York, pp. 185–212. [Google Scholar]
- Spinelli S, Schwandt ML, Lindell SG, Newman TK, Heilig M, Suomi SJ, Higley JD, Goldman D, Barr CS, 2007. Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Dev. Psychopathol 19, 977–987. [DOI] [PubMed] [Google Scholar]
- Sussman AF, Ha JC, Bentson KL, Crockett CM, 2013. Temperament in rhesus, long-tailed, and pigtailed macaques varies by species and sex. Am. J. Primatol 75, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sysoeva O, Kulikova M, Malyuchenko N, Tonevitskii A, Ivanitskii A, 2010. Genetic and social factors in the development of aggression. Hum. Physiol 36, 40–46. [PubMed] [Google Scholar]
- Trefilov A, Berard J, Krawczak M, Schmidtke J, 2000. Natal dispersal in rhesus macaques is related to serotonin transporter gene promoter variation. Behav. Genet 30, 295–301. [DOI] [PubMed] [Google Scholar]
- Vaillancourt T, 2013. Do human females use indirect aggression as an intrasexual competition strategy? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 368, 20130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona E, Joiner TE, Johnson F, Bender TW, 2006. Gender specific gene-environment interactions on laboratory-assessed aggression. Biol. Psychol 71, 33–41. [DOI] [PubMed] [Google Scholar]
- Virkkunen M, Eggert M, Rawlings R, Linnoila M, 1996. A prospective follow-up study of alcoholic violent offenders and fire setters. Arch. Gen. Psychiatry 53, 523–529. [DOI] [PubMed] [Google Scholar]
- Walderhaug E, Herman AI, Magnusson A, Morgan MJ, Landrø NI, 2010. The short (S) allele of the serotonin transporter polymorphism and acute tryptophan depletion both increase impulsivity in men. Neurosci. Lett 473, 208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, Suomi SJ, 2006. Differential functional variability of serotonin transporter and monoamine oxidase a genes in macaque species displaying contrasting levels of aggression-related behavior. Behav. Genet 36, 163–172. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ, Higley JD, Mehlman PT, 1999. CSF 5-HIAA and aggression in female macaque monkeys: species and interindividual differences. Psychopharmacology 146, 440–446. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ, Chavanne TJ, Houser L, Hurley A, Cleveland A, Snoy PJ, Higley JD, 2003. Physiological correlates of aggression and impulsivity in free-ranging female primates. Neuropsychopharmacology 28, 1045–1055. [DOI] [PubMed] [Google Scholar]
- Wilson M, Daly M, 1985. Competitiveness, risk taking, and violence: the young male syndrome. Ethol. Sociobiol 6, 59–73. [Google Scholar]
- Wilson ME, Gordon TP, 1979. Sexual activity of male rhesus monkeys introduced into a heterosexual group. Am. J. Phys. Anthropol 50, 515–523. [DOI] [PubMed] [Google Scholar]
- Witt R, Schmidt C, Schmitt J, 1981. Social rank and darwinian fitness in a multimale group of barbary macaques (Macaca sylvana linnaeus, 1758). Dominance reversals and male reproductive success. Folia Primatol. 36, 201–211. [DOI] [PubMed] [Google Scholar]
- Wood EK, Gabrielle N, Hunter J, Skowbo AN, Schwandt ML, Lindell SG, Barr CS, Suomi SJ, Higley JD, 2021. Early rearing conditions affect monoamine metabolite levels during baseline and periods of social separation stress: a non-human primate model (Macaca mulatta). Frontiers in Human Neuroscience 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangham R, 1981. Drinking competition in vervet monkeys. Anim. Behav 29, 904–910. [Google Scholar]
- Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE, 2009. Male dominance rank and reproductive success in chimpanzees, pan troglodytes schweinfurthii. Anim. Behav 77, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Mohr C, Spangler G, 2009. Genetic and attachment influences on adolescents’ regulation of autonomy and aggressiveness. J. Child Psychol. Psychiatry 50, 1339–1347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As the data are part of a larger database that is not owned by the primary authors, we note in the manuscript that the data are available on request.


