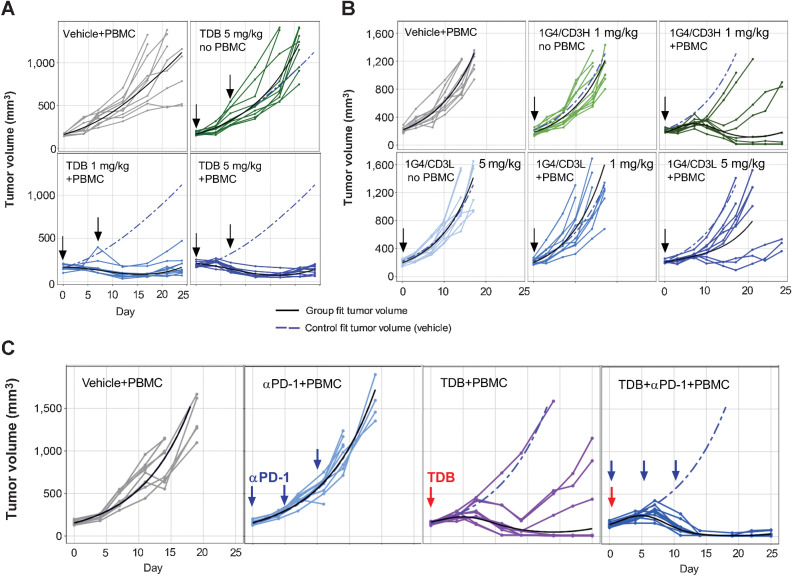
Figure 6.
LY6G6D-TDB is efficacious in inhibiting tumor growth in established xenograft tumor models in NSG mice. A,In vivo efficacy of 1G4/CD3H in an established LS1034 xenograft tumor model. After tumor establishment, mice received intravenous injections on days 0 and 7 of the following: (i) vehicle; (ii) 1G4/CD3H at 5 mg/kg; (iii) 1G4/CD3H at 1 mg/kg; (iv) 1G4/CD3H at 5 mg/kg. n = 10 per group B, Comparison of 1G4-TDBs with high- or low-affinity CD3 arm in established HT55 xenograft tumor model. After tumor establishment, mice received intravenous injection of either 1G4/CD3H at 1 mg/kg, or 1G4/CD3L at 1 mg/kg or 5 mg/kg on day 0. Control animals received either vehicle, or 1G4/CD3L at 5 mg/kg (without PBMCs), as labeled in the panel. n = 9 per group. C, Combination of 1G4/CD3H and anti–PD-1 antibody in established HT55 xenograft tumor model. After tumor establishment, mice received intravenous injection of the following: (i) vehicle; (ii) anti–PD-1 antibody at 10 mg/kg on days 0, 7, and 14; (iii) 1G4/CD3H at 1 mg/kg on day 0; (iv) co-administration of 1G4/CD3H at 1 mg/kg and anti–PD-1 antibody at 10 mg/kg on day 0, anti–PD-1 antibody on days 7 and 14. Some of the mice were euthanized before the end of the study due to tumor volumes ≥2,000 mm3 or losses in body weight ≥20% from their weight at the start of treatment per IACUC guidelines.