Figure 3.
RET::GRB2 fusion is oncogenic and sensitive to clinical grade RET inhibitors
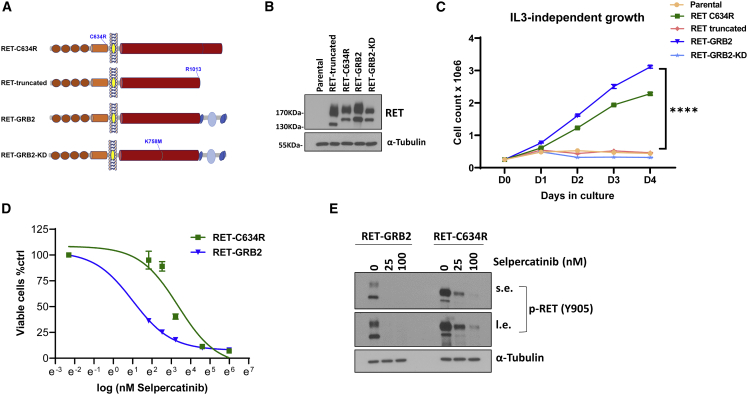
(A) Constructs used to evaluate transforming activity in Ba/F3 cells: (domains are depicted as in Figure 2A); RET-C634R, pathogenic RET mutant; RET truncated, first 1,013 amino acids of RET, containing only the RET component of the fusion; RET::GRB2, contains the RET::GRB2 fusion; RET::GRB2-KD, contains the kinase-dead version of the RET::GRB2 fusion carrying a K758 M mutation in the RET catalytic domain.
(B) Western blot of lysates from Ba/F3 cells stably expressing the constructs indicated in (A), along with parental cells, probed for total RET, and α-tubulin as a loading control; these experiments were repeated 3 times.
(C) Growth rate of Ba/F3 cells stably expressing the constructs indicated in (A) and (B), and parental cells cultured in the absence of interleukin 3 (IL-3). Cells were plated in triplicate and counted daily for 4 days; experiments were repeated 3 times. ∗∗∗∗p < 0.0001, two-way ANOVA.
(D) IC50 concentration-response curves to selpercatinib at doses of 0, 6.25, 12.5, 25, 50, 100, and 400 nM for 72 h measuring inhibition of growth of Ba/F3 cells expressing the RET::GRB2 fusion (4.1 nM, 95% CI, 3.4–4.9 nM) or RET-C634R mutant (29.9 nM, 95% CI, 21.2–42.6 nM) seeded in triplicate per dose and repeated three times.
(E) Lysates from Ba/F3 cells expressing RET::GRB2 or RET-C634R treated with 25 or 100 nM selpercatinib or vehicle for 4 h were probed with P-RET (Y905), and α-tubulin as loading control; three biological replicates were performed. s.e. and l.e. indicate short and long immunoblot exposure, respectively.