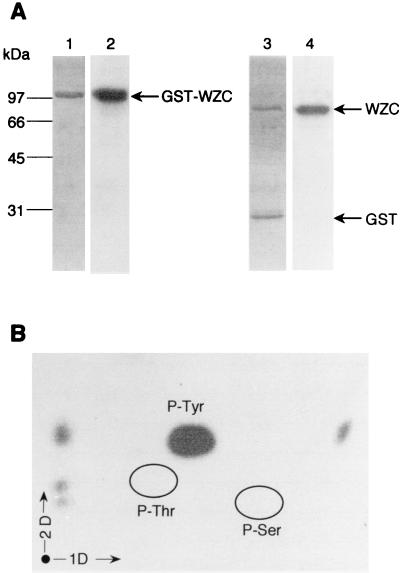
FIG. 3.
GST-Wzc autophosphorylation assay. About 3 μg of purified GST-Wzc was incubated with [γ-32P]ATP. The protein was analyzed by SDS-PAGE; gels were soaked in 16% TCA and either stained with Coomassie blue (lane 1) or submitted to autoradiography (lane 2). The protein was then hydrolyzed by thrombin and analyzed by SDS-PAGE. The products of hydrolysis were revealed by Coomassie blue staining (lane 3) or autoradiography (lane 4). (B) Phosphoamino acid content of GST-Wzc. GST-Wzc labeled with [γ-32P]ATP was analyzed by SDS-PAGE, electroblotted onto an Immobilon PVDF membrane, excised, and hydrolyzed in acid. The phosphoamino acids thus liberated were separated by electrophoresis in the first dimension (1D) and ascending chromatography in the second dimension (2D). After migration, radioactive molecules were detected by autoradiography. Authentic phosphoserine (P-Ser), phosphothreonine (P-Thr), and phosphotyrosine (P-Tyr) were run in parallel and visualized by ninhydrin staining.